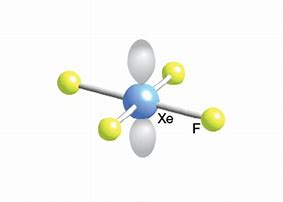
Difference between shape and structure of XeF4???
1 Answer
Feb 27, 2018
Between molecular shape and electronic structure?
Explanation:
We gots
The remaining four electrons are conceived to reside in two xenon-centred lone-pairs....and simple VESPER that ALL the electron pairs assume an octahedral geometry around the xenon centre.... However we describe molecular geometry on the basis of bonding pairs...and thus while electronic geometry is OCTAHEDRAL, molecular geometry is SQUARE PLANAR...with all