Do ionic compounds have molecular geometry?
1 Answer
Nov 21, 2017
No, but any polyatomic ions in them have a molecular geometry.
Explanation:
For example, nitrate ions
The ions in ionic compounds arrange themselves in characteristic repeating patterns called lattice structures.
The smallest possible repeating unit is called a unit cell.
Here is a diagram of a unit cell for
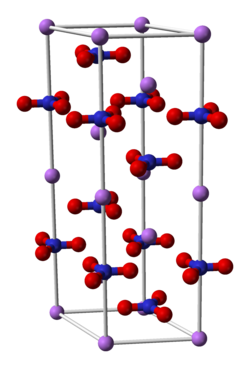
We see the trigonal planar nitrate ions and the purple

