Do noble gases have high or low ionization energies?
1 Answer
Dec 12, 2016
Ionization energies increase across a Period from left to right as we face the Table............
Explanation:
.........And ionization energies decrease down a Group.
Thus Noble Gases have exceptionally high ionization energies.
Simply considerations of electrostatics should rationalize this trend. As nuclear charge increases in the same Period, ionization energy progressively increases. It reaches a maximum when the valence electronic shell is complete.
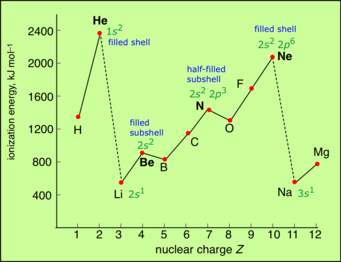
This diagram is well-used, and illustrative.

