Do solid particles vibrate?
1 Answer
Yes. And in lattices, if the atoms themselves are heavier, they vibrate at lower natural frequencies, and vice versa.
This can be modeled by an infinite array of harmonic oscillators, i.e. balls and springs.
For simplicity, consider a segment of a 1D lattice of
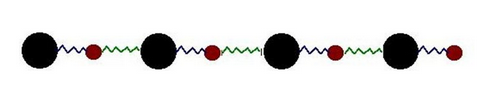
The position over time for the
#x_j(t) = A_(kappa) e^(i(omega_(kappa)t + kappa aj))#
They would vibrate with an angular frequency
#omega_(kappa) = 2sqrt(k/m)|sin((kappa a)/2)|#
and, since
#omega_D = 2sqrt(k/m)# ,where:
#k# is the force constant between the two identical atoms in#"kg/s"^2# .#kappa# is the wave number (the number of waves for a unit distance).#m# is the mass of each atom in#"kg"# .#a# is the position coordinate of a given atom.
Above
In other words, the wavelength

