Does 2-methyl-3-hexanone exhibit hydrogen bonding?
'Describe the difference in intermolecular forces between 2-methyl-3-hexanone and 5-methyl-3-hexanol and how this affects the properties of the substances.'
I'm looking at the molecules on Chemspider, and I want to say that because 5-methyl-3-hexanol has a hydroxyl group, it will form hydrogen bonds, whereas 2-methyl-3-hexanone wouldn't. That in turn means 5-methyl-3-hexanol has higher melting and boiling points. Does that sound reasonable, or am I missing something?
'Describe the difference in intermolecular forces between 2-methyl-3-hexanone and 5-methyl-3-hexanol and how this affects the properties of the substances.'
I'm looking at the molecules on Chemspider, and I want to say that because 5-methyl-3-hexanol has a hydroxyl group, it will form hydrogen bonds, whereas 2-methyl-3-hexanone wouldn't. That in turn means 5-methyl-3-hexanol has higher melting and boiling points. Does that sound reasonable, or am I missing something?
1 Answer
2-methyl-3-hexanone would not exhibit hydrogen-bonding with itself... but it would with 5-methyl-3-hexanol. Either way, 5-methyl-3-hexanol should hypothetically have the higher melting and boiling point.
A simpler example is that acetone can hydrogen-bond with water, but not with itself.
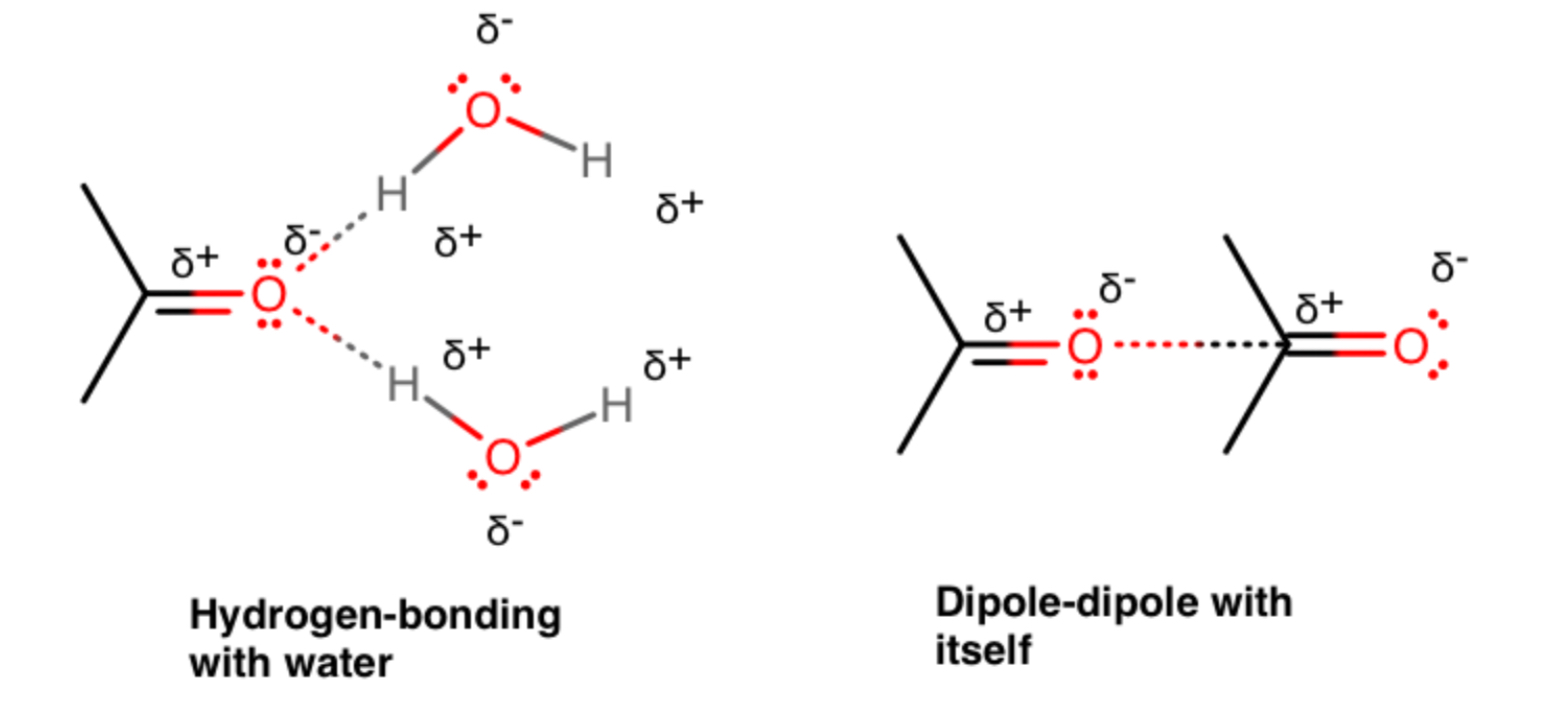
What matters is how it interacts with itself.
So yes, 5-methyl-3-hexanol does hydrogen-bond with itself and should have the higher melting and boiling point.

