Does a leaving group affect the reaction rate?
2 Answers
Explanation:
Nucleophilic substitution reactions whether it is SN1 or SN2 favour better leaving group.
With a better leaving group, the substitution occurs faster.
This also applies to elimination reactions E1 and E2.
Usually yes, but not necessarily. It depends on which mechanistic step is the rate-determining step and whether that leaving group leaves during the rate-determining step or not. It tends to be that it does, though.
Let's suppose we have an
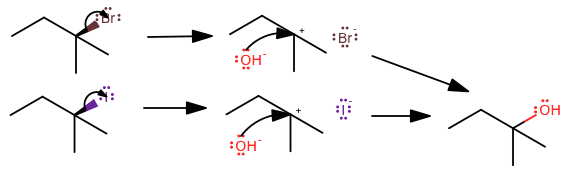
In this case, the amount of steric hindrance and carbocation stability makes it so the leaving group leaves on the rate-determining step. The tertiary carbocation intermediate is the most stable of all the alkyl carbocations
The second reaction is faster because iodide is the largest stable halogen, therefore forming a weaker bond, and speeding up the rate-determining step. It's a weaker base because its acid is
Let's suppose we had a situation where the leaving group participates in a reaction step that is NOT the rate-determining step.
(This may feel intuitive after you read it, but may not be entirely obvious beforehand.)
If you had an
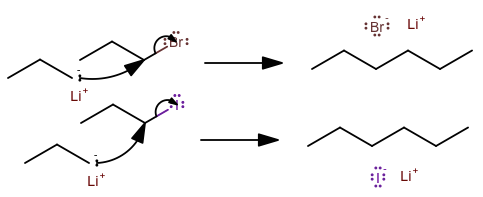
Here the rate-determining step is the nucleophilic attack, NOT the step where the leaving group leaves (because the alkyllithium reagent is very quick, the reaction has no detectable intermediate).
In either of these two reactions, the difference in reaction rates is insignificant. The change in leaving group here does not affect the reaction rate substantially---it's negligible.


