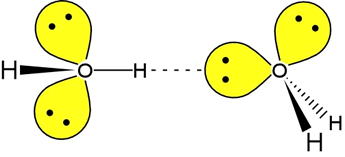
Does hydrogen bonding have a directional character?
1 Answer
Yes, hydrogen bonding does have directional character.
Explanation:
Hydrogen bonds arise, in part, from the high electronegativity differences between the hydrogen atom and an atom of one of the elements fluorine, oxygen, or nitrogen. Since the
The second vital requirement for hydrogen bonding to take place is the presence of a lone pair on the local species. The negative charge of the lone pair acts in a direction away from the atom with which it is associated.
With both of these factors combined it becomes easier to see that, since like charges attract, the strength of the hydrogen bond will be strongest when these two forces act in directions that are as different as possible, which is the case when the original electronegative atom, the hydrogen atom, the atom with lone pair, and the lone pair itself, are all aligned with one another.