Electron configuration?
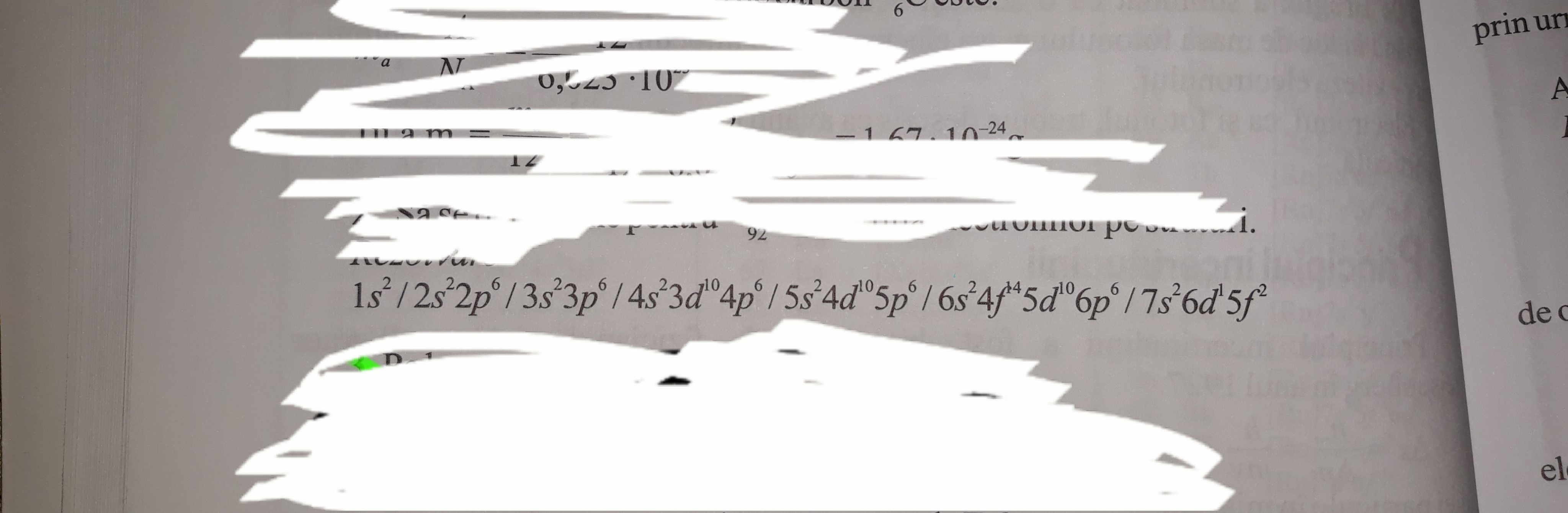
This is the electron configuration of U_{92} I found in a book. What do they want to say by using "/"?
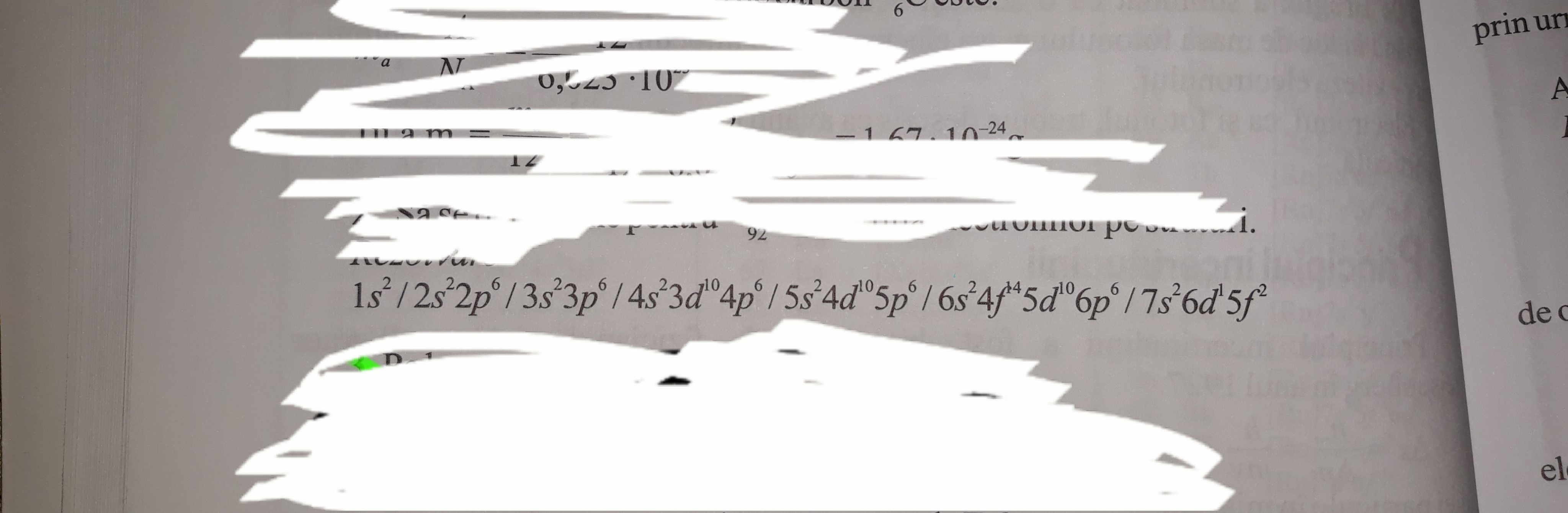
This is the electron configuration of
1 Answer
Jun 24, 2018
See carefully they are indicating where a period in the Periodic table ends and how many elements are there in that period
Explanation:
before first"/" there are two electron which means there are total no. two elements in that period.
similarly in second and third "/" it shows that this period has 8 elements..........like this it goes on to the group and period where
as it says it is in 7th period and third group(we are not adding the f electrons as they (f- block) belong to the same group)