[Equilibrium] Does my book contradict itself?
In an example, it shows that #K# for the reaction #N_2 + 3H_2 rightleftharpoons 2NH_3# is #3.8 xx 10^4# at #127^@C# . (They give you the concentrations of reactants.)
Then it shows that #K# for the equation #1/2 N_2 + 3/2H_2 rightleftharpoons NH_3 # is #1.9 xx 10^2# because it's equal to K from the above part, raised to the #1/2# power.
However, later, it says this:
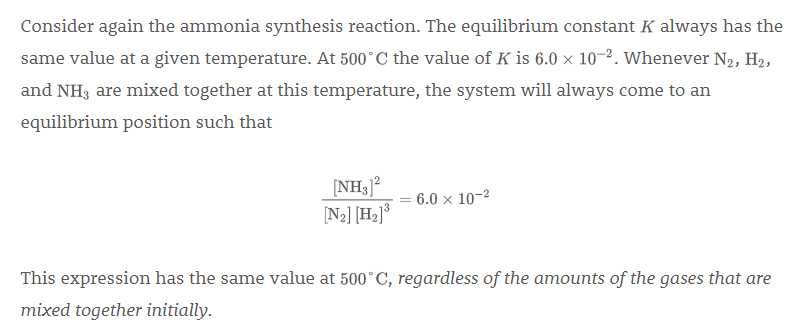
I'm confused about the last sentence. If the equilibrium constant is the same regardless of the amounts of the gases, then why does #K# differ in the example above?
In an example, it shows that
Then it shows that
However, later, it says this:

I'm confused about the last sentence. If the equilibrium constant is the same regardless of the amounts of the gases, then why does
1 Answer
See Below
Explanation:
Ok, right off the bat, Equilibrium constants depend on temperature (since they are equal to the forward rate constant divided by the reverse..and those are temp dependent). Another factor that affects equilibrium values is the balancing of the chemical equation. That is why your book has those different K values with different balanced equations.
As for the concentration of reactants and products, let me explain it like I did to my class the other day. Using the idea of a half-pipe for snowboarding (Olympics were on TV). Left side is reactants, right is products.
If you have a halfpipe that is on level ground, then the equilibrium will be 1....meaning it doens't matter where you start on the half-pipe, up the left wall or the right wall, you'll slide down to the center and undulate there at the center like a pendulum. This is a reaction in which products and reactants are equally favored.
If you take the half-pipe and you place it on tilted ground, say with the right side 10 feet higher. This will be a reaction that is product favored (you'll end up at the bottom and this will be on the left). if you start on the left wall, you'll still slide forward, but you'll end up pendulum-ing on the left side....and if you start on the right side, you'll slide to the left side and end up pendulum-ing on the left......this is a reactant favored reaction - it doesn't matter where you start, you'll end up in the same spot (with the same ratio or Right/Left....or Products/Reactants).
Repeat the half-pipe on tilted ground, but this time with the Left Side raised by 10 feet..and you'll end up pendulum-ing on the right side (product favored).
The K value is a constant that represents (among other things) the ratio of your products to your reactants (and raised to their powers). So since it is a constant, you can start with All Products...and you'll get to the K value. You can start with All Reactants...and you'll get to the K value.....you can start with half and half..and you'll get to the K value.
Weird analogy - hope it helps.
