Ethyl Propene?
When do you use numbers? THANK YOU I actually sent the question without the photo last time.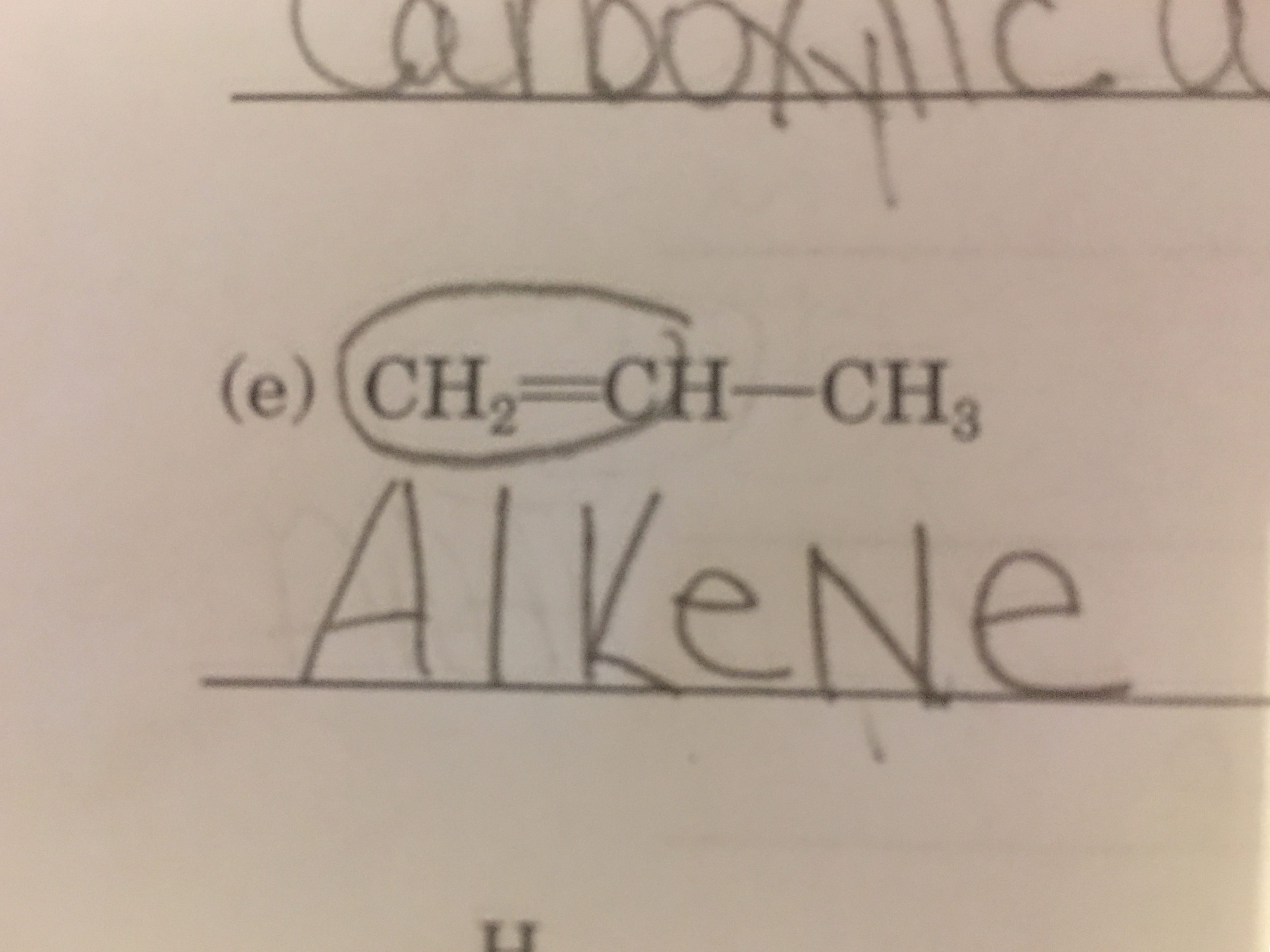
When do you use numbers? THANK YOU I actually sent the question without the photo last time.
1 Answer
Mar 31, 2018
See below
Explanation:
That's an alkene as you stated...
but I don't see an ethyl group, remember an ethyl group is a branch of the main chain of carbon
That's a propene
You don't need a
When naming in this case, you know that the double bond gets the priority, so when I start naming I can make sure in this case, that the double bond is always on the first Carbon. So a prop-2-ene does not exist

