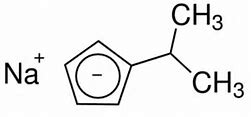
Explain how the following two compounds can have the same conjugate base. Is this conjugate base aromatic?
1 Answer
Feb 6, 2018
What is the Huckel criterion for aromaticity....?
Explanation:
The Huckel criterion specifies
 sigmaaldrich.com
sigmaaldrich.com
And this is a commonly used ligand in organometallic chemistry....

