Explain.(i)Pentane is distilled off first when a liquid mixture containing pentane,heptane and nonane is heated.(ii)Hexane floats on water. (iii) cyclopentane may be easily separated from an aqueous salt solution?
1 Answer
In every case, the alkanes have weaker intermolecular forces of attraction.
Explanation:
Part (i)
Here we have linear alkanes with different chain lengths.
The longest alkane will have the strongest London dispersion forces of attraction, because there will be more points at which the chains can interact.
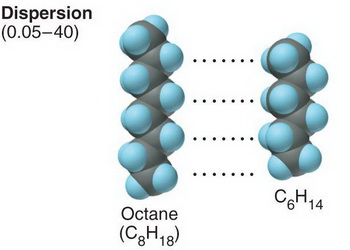
Pentane will have the weakest attractive forces, followed by heptane, and nonane will have the strongest.
Pentane will boil first (36 °C), followed by heptane (98 °C), and then by nonane (151 °C)
Part (ii)
Hexane has only weak London dispersion forces of attraction, while water has strong hydrogen bonding attractions.
If we add hexane to water, the hexane will float on the top of the water with no apparent mixing.
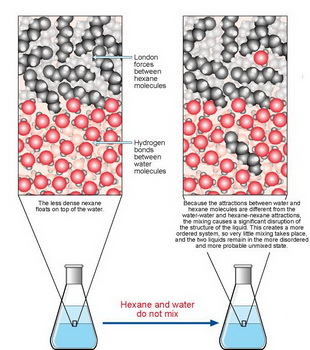
(from www.mpcfaculty.net)
The only attractive forces among the hexane and water molecules are London forces.
Thus, a few hexane molecules will enter the water layer, but the strong attractive forces among the water molecules keeps most of the hexane molecules out.
Similarly, a few water molecules will enter the hexane layer because of the water-hexane London forces.
Also, the strong attractive forces among the water molecules holds the water molecules close together in a compact volume.
The water is denser than the hexane, so the hexane floats on top.
Part (iii)
"We leave it as an exercise for the student" to answer Part (iii).
Hint: The explanation is similar to that of Part (ii).

