Explain oxygen is paramagnetic while c2 is diamagnetic?
1 Answer
Mar 23, 2018
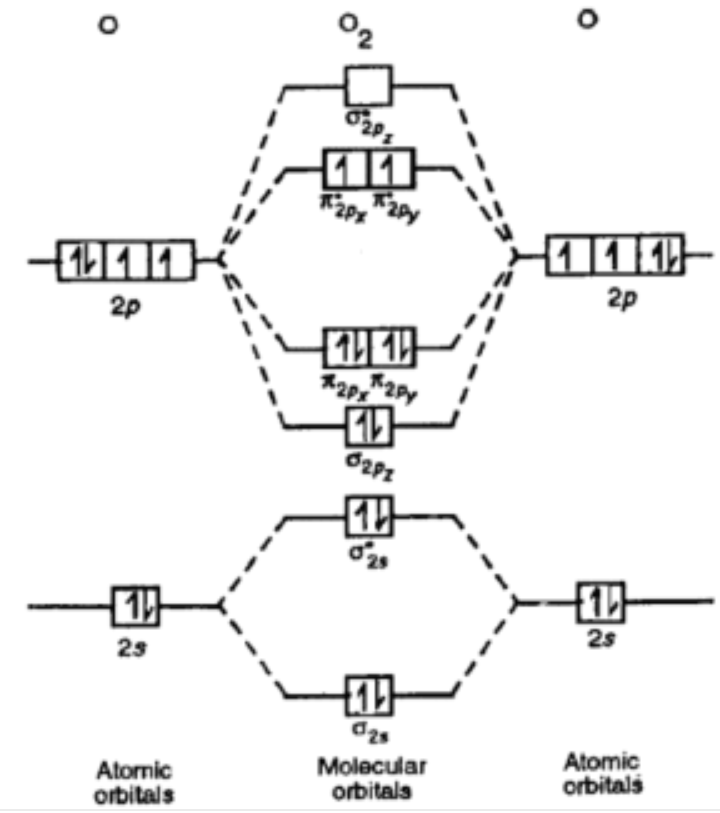
Explanation:
Electrons, besides being around the atom in their respective orbitals, also have a spin angular momentum, which could interact with an external magnetic field; unpaired electrons have the same spin, which allows for interaction with the magnetic field.

