Explain the origin of diamagnetism and paramagnetism?
1 Answer
Diamagnetism and Paramagnetism arise out of electron filling of elements.
Whenever two electrons are paired together in an orbital, or their total spin is
These element are also called non-magnetic in layman's term. Water, wood, most of organic compounds belong to this category.
Diamagnetic materials are repelled by a magnetic field. An applied magnetic field in these materials creates an induced magnetic field in an opposite direction. Thus, causing a repulsive force.
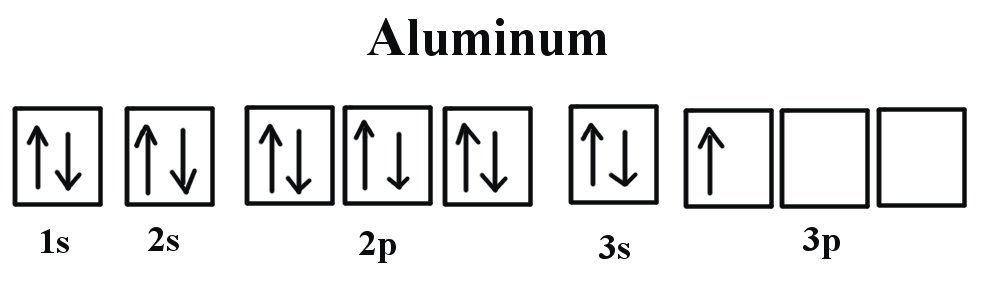
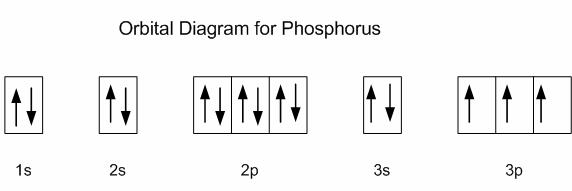
An unpaired electron is called paramagnetic electron. Atom with one or more unpaired electrons is termed as paramagnetic. For example see figures of electron filling of Aluminum and Phosphorus below. Iron oxide (FeO) is also paramegnetic material.
Paramagnetism is a type of magnetism where certain materials are weakly attracted by a magnetic field applied externally. These form induced magnetic fields in the direction of the applied magnetic field.
As such these are weak magnetic. In the absence of an applied field the constituent atoms or molecules of paramagnetic materials have permanent magnetic moments due to unpaired electrons.