Let me first quickly go over a little bit about #color(red)"Carbohydrate chemistry"# and then I will get to the answer.
#color(red)"Lactose"# and #color(red)"sucrose"# are both carbohydrates or more commonly referred to as sugars. More specifically, they are #color(red)"disacharrides"# as they are both made up of 2 sugar units.
#color(blue)["Figure 1. The disacharrides Sucrose and Lactose in their ring forms"#

#color(white)#
#color(blue)[---------------------#
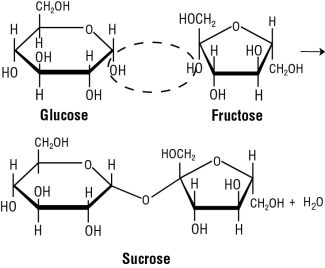
Let's look at sucrose first. Sucrose is made by the condensation reaction of a #color(red)"glucose molecule"# and a #color(red)"fructose molecule"#. What essentially happens is the hemiacetal of the glucose molecule reacts with the hydroxyl group of the fructose molecule. This reaction ends up forming an #color(red)"O-glycosidic bond"#. [#color(blue)["Figure 2"#] (a reaction you should be familiar with from organic chemistry)
#color(blue)["Figure 2: Sucrose formation"#
#color(blue)[---------------------#
#color(white)(a)#
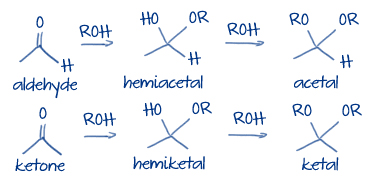
Figure 3 below shows a general hemiacetal/hemiketal and acetal/ketal reaction.
#color(blue)["Figure 3: Hemiacetal/hemiketal and acetal/ketal general reaction"#
#color(blue)[---------------------#
#color(white)(a)#
During the formation of sucrose, both the glucose and fructose molecules' anomeric carbons get involved to form the O-glycosidic bond. An #color(red)"anomeric carbon"# is the carbon that was once a part of the carbonyl group of the sugar molecule before it reacts internally with its own hydroxyl group. This reaction eventually leads to the formation of the cyclic sugar.
#color(blue)["Figure 4: Anomeric carbon of Glucose"#
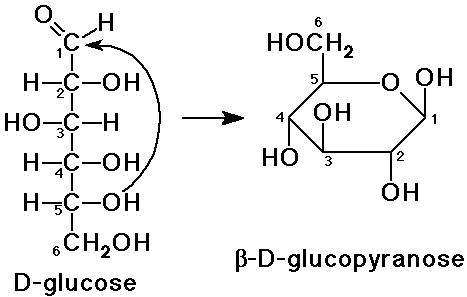
Looking at the straight chain form of glucose on the left side of the figure, the carbonyl carbon (C1) reacts internally with the hydroxyl group - the one attached to C5 - to form a 5 membered ring (the right side of the figure). The carbon labeled 1 is the anomeric carbon
#color(blue)[---------------------#
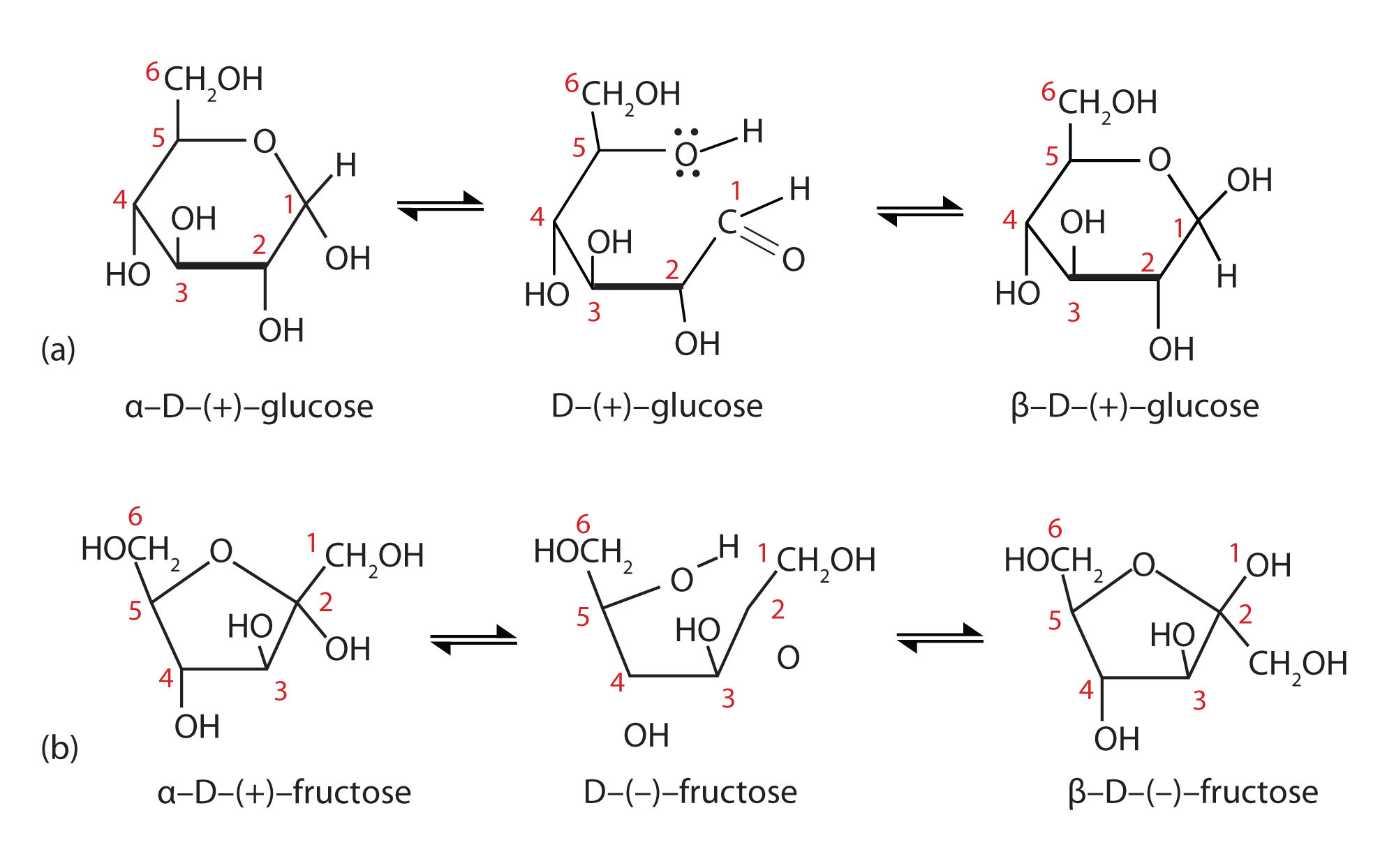
The cool thing about anomeric carbons is that depending on their stereochemistry two #color(red)"anomers"# (isomers) can exist - the #color(red)[alpha]# or the #color(red)[beta]# form. At equilibrium, the 2 anomers and the straight chain form will exist with the #beta# form predominating in solution. These two forms can interconvert spontaneously in a process called #color(red)"mutarotation".#
#color(blue)["Figure 5: Mutarotation where equilibrium exists between cyclic"#
#color(blue)["and straight chain forms of the sugars"#
#color(blue)[---------------------#
#color(white)(a)#
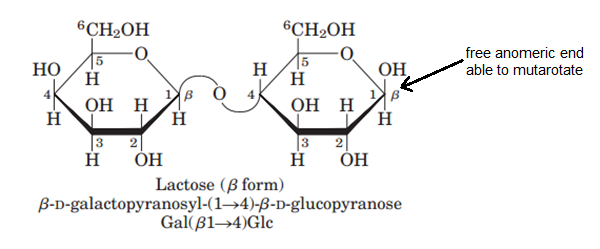
In order for mutarotation to occur, however, there needs to be a #color(red)"free anomeric carbon end"# or an anomeric carbon that is not involved in any bond. If the anomeric carbon is not involved in any kind of bond, it is free to equilibriate to its straight chain or 2 cyclic isomeric forms.
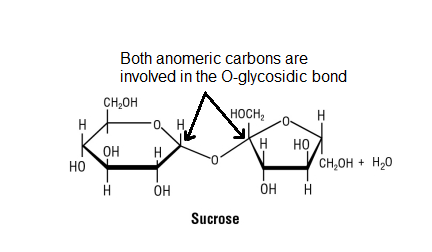
Well, sucrose can't freely equilibriate as its 2 anomeric carbons are already involved in the glycosidic linkage.
#color(blue)["Figure 6: Sucrose has no available anomeric carbon"#
#color(white)#
Lactose on the other hand, can mutarotate since it does have an available anomeric carbon.
#color(blue)["Figure 7: Lactose and its free anomeric end"#