For Enthalpy? the formula and what answer you all got? The sum(product)-The sum(reactant)
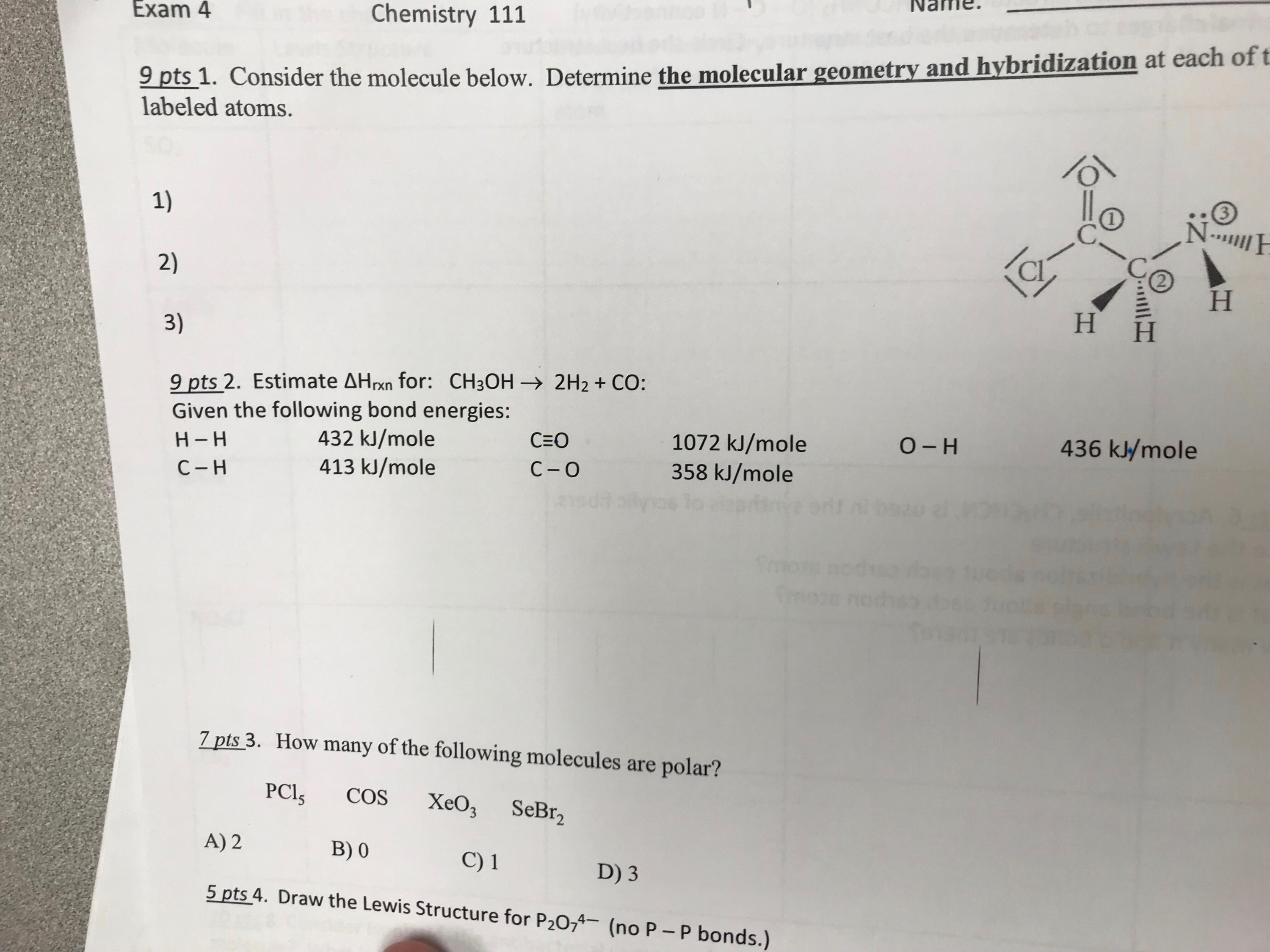
1 Answer
Dec 4, 2017
Methanol,

decomposes into hydrogen (
We're breaking three
Forming bonds is exothermic, and breaking bonds is endothermic, so with using your data,
where b and f indicate bonds broken and bonds formed, respectively. Hence
is the enthalpy of this reaction, which is energetically unfavorable.

