For the following pairs of electron transition, which produces the emission with longest wavelength. give rationale behind your answer? (a) n=3--> n=1 versus n=2 --> n=1 (b) 3p --> 2s versus 2p-->1s
1 Answer
In (a) the greater energy is for n=3 to n=1; for (b) the greater energy is 2p to 1s.
Explanation:
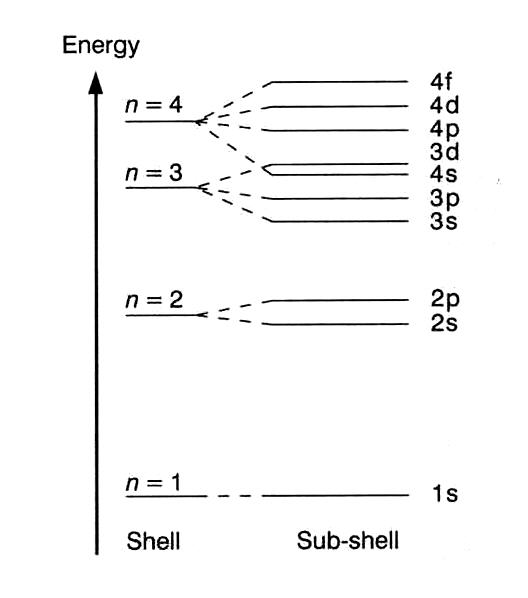
If one calculates the energy levels in a hydrogen atom, it is seen that greater values of n are associated with higher energy levels. When an electron drops from one level to another, the greater the change in n (assuming both transitions end at the same level, as is the case here), the greater the reduction in energy.
For the second case, one needs to know that the differences between consecutive values of n get smaller and smaller as n increases. So, there is a much smaller difference between n=3 to n=2 than between n=2 and n=1.
For hydrogen, 2p and 2s are equal in energy, so it is valid to just consider the change in n. For larger atoms, s and p subshells separate slightly in energy, but not nearly enough to compare to the difference between shells. So, the change from 3p to 2s is only slightly greater than the change from 3s to 2s.
This diagram may clear up the ideas a bit