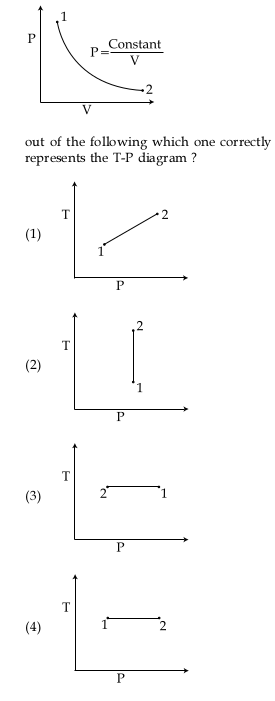
For the P-V diagram given for an ideal gas, the T-P graph is=?
2 Answers
Mar 28, 2018
3)
Explanation:
The curve is an isotherm
Mar 28, 2018
Explanation:
The ideal gas law is:
If we write
Look at the