For the questions, use the image below What energy corresponds to the electron affinity of fluorine? What energy corresponds to the 1st ionization energy of potassium? Which energy change corresponds to the negative lattice energy of potassium fluoride?
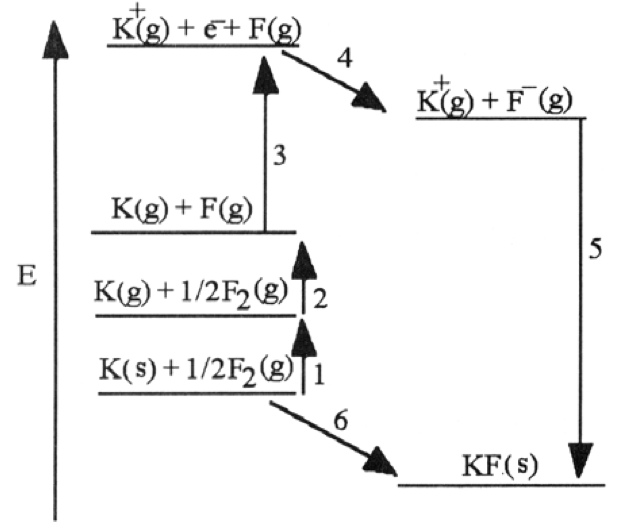
Answer using the numbers 1, 2, 3, 4, 5, or 6 from the chart.

Answer using the numbers 1, 2, 3, 4, 5, or 6 from the chart.
1 Answer
Nov 18, 2017
Here's what I get.
Explanation:
(a). Electron affinity of fluorine
(b). 1st ionization energy of potassium
(c). Negative lattice energy of potassium fluoride

