Gibbs Free Energy. Why are equilibrium constants for a chemical reaction temperature dependent?
1 Answer
Because rate constants are temperature-dependent, and by changing the temperature, you change the ratio of rate constants so that
Although you may be familiar with this in the context of this equation:
#DeltaG^@ = -RTlnK#
That isn't actually where the temperature dependence is readily seen. Consider the Arrhenius equation:
#k = Ae^(-E_a//RT)# where
#k# is the rate constant at temperature#T# ,#A# is the frequency factor of the reaction (i.e. the limiting rate constant at high temperature), and#E_a# is the activation energy.
Now, take two temperatures and two rate constants, assuming
#k_2/k_1 = "exp"(-E_a/R[1/T_2 - 1/T_1])#
#=> color(green)(ln(k_2/k_1) = -E_a/R[1/T_2 - 1/T_1])# where
#"exp"(x) = e^x# .
We currently have the relationship for two rate constants each at different temperatures. Now, think about where activation energy is on a reaction coordinate diagram:
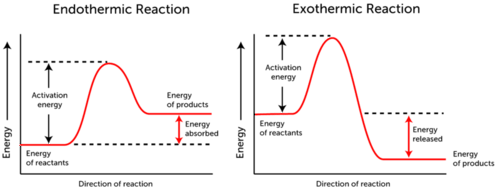
We see that
So now, consider that the rate of the forward reaction equals the rate of the reverse reaction at equilibrium. Take the reaction as:
#aA + bB rightleftharpoons cC + dD#
The rate laws would be:
#r_(fwd)(t) = k_(fwd)[A]^a[B]^b#
#r_(rev)(t) = k_(rev)[C]^c[D]^d#
So we set them equal:
#k_(fwd)[A]^a[B]^b = k_(rev)[C]^c[D]^d#
By definition, the equilibrium constant is:
#=> K -= ([C]^c[D]^d)/([A]^a[B]^b) = (k_(fwd))/(k_(rev))#
Now, take
#K_1 = (k_(1,fwd))/(k_(1,rev))# #" "" "# #K_2 = (k_(2,fwd))/(k_(2,rev))#
Substitute back into the Arrhenius equation for the forward and reverse reactions.
#ln(k_(2,fwd)/k_(1,fwd)) = -E_(a,fwd)/R[1/T_2 - 1/T_1]#
#ln(k_(2,rev)/k_(1,rev)) = -E_(a,rev)/R[1/T_2 - 1/T_1]#
Use the fact that
#ln(k_(2,fwd)/k_(1,fwd)) - ln(k_(2,rev)/k_(1,rev))#
#= ln(k_(2,fwd)/k_(1,fwd) cdot 1/(k_(2,rev)//k_(1,rev)))#
#= ln((k_(2,fwd)//k_(2,rev)) / (k_(1,rev)//k_(1,fwd)))#
#= ln(K_2/K_1)#
The right-hand side by subtraction becomes:
#-E_(a,fwd)/R[1/T_2 - 1/T_1] - (-E_(a,rev)/R[1/T_2 - 1/T_1])#
#= -(E_(a,fwd) - E_(a,rev))/R[1/T_2 - 1/T_1]#
But look up at the reaction coordinate diagram above. We can make the connection that
Therefore, we arrive at the van't Hoff equation for equilibrium constants vs. temperature:
#color(blue)(ln(K_2/K_1) = -(DeltaH_(rxn))/R[1/T_2 - 1/T_1])# which looks strikingly like the Arrhenius equation (not a coincidence!).
Or put in another form,
#color(blue)(ln K = -(DeltaH_(rxn))/R 1/T + (DeltaS_(rxn))/R)#
Hence, we have shown that equilibrium constants certainly are temperature-dependent.
Endothermic reactions have increased

