Given the balanced ionic equation representing a reaction below. In this reaction, where are electrons transferred from?
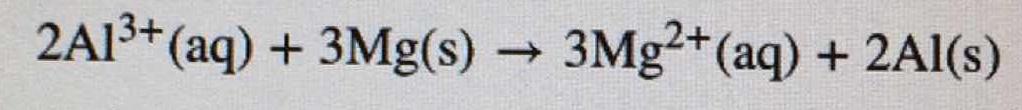
2 Answers
This is a redox reaction in which electrons are formally transferred....
Explanation:
And we cross multiply the individual redox equations so that electrons are absent from the final redox equation, i.e.
And finally.............
The electrons are transferred from the magnesium metal solid to the Aluminum ions
Explanation:
The Aluminum is reduced as it charge goes from + 3 to zero, as the Al gains electrons. That means the charge or oxidation number of aluminum goes down. The Aluminum acts as an oxidizing agent in this reaction.
The Magnesium is oxidized as it charge goes from zero to +2 . The magnesium has lost electrons. This causes the charge or oxidation number magnesium to become more positive. The Magnesium acts as a reducing agent in this reaction.
Electrons are transferred from the Magnesium solid to the Aluminum ion.

