Guys, how do I make m-nitrobenzoic acid if I have benzene as a starting compound?
1 Answer
From benzene, there's not much you can do for the first step other than bromination, or Friedel-Crafts. But using Friedel-Crafts is probably something you'd do if you're feeling extra creative.
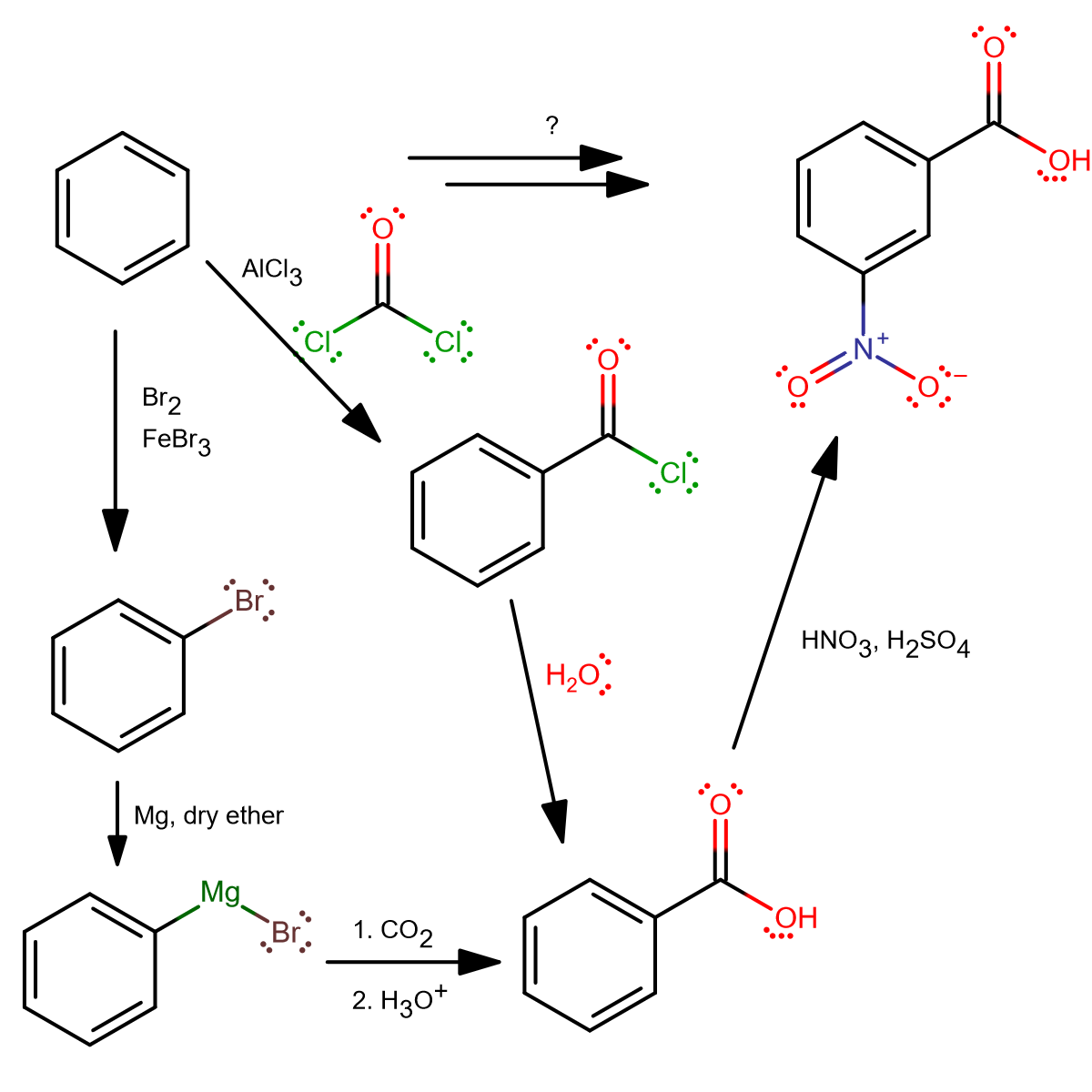
From above, we see two ways (there may be others).
FIRST STEP: BROMINATION
Four/Five steps.
Most people may think of bromination as the first step, because it's usually the first benzene-based reaction they learn. In that case, to turn bromobenzene into a nucleophile, one could add magnesium in dry ether to form a Grignard reagent.
This reacts well with
FIRST STEP: FRIEDEL-CRAFTS ACYLATION
Three steps.
This may not be your first thought, but... Friedel-Crafts acylation using phosgene! It generates benzoyl chloride, which readily reacts with water to form benzoic acid. From there, it's a straightforward nitration, and as before, the carboxyl group directs the nitronium cation to the meta position.

