Help me to solve this question please?
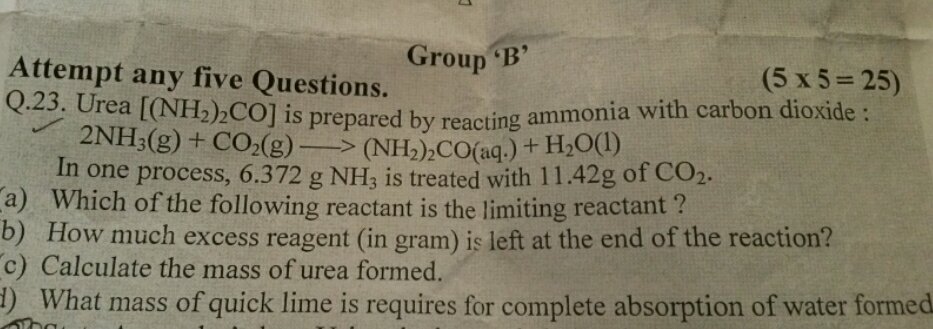
1 Answer
Convert mass to moles, realize that ratio of ammonia to carbon dioxide is 2:1 so ammonia will be the limiting reactant. Since 2 moles of ammonia react with one mole of
Explanation:
Since 2 moles of ammonia react with one mole of
One mole of urea is formed for each mole of

