Help with question 11 c guys ?
1 Answer
DISCLAIMER: LONG ANSWER!
The idea here is to use two situations and conservation of mass to separately determine the mass percent of two out of three components in thiophene, which we denote as
- Find mass percent of
#"S"# in thiophene. - Find mass percent of
#"C"# in thiophene and use that to deduce mass percent of#"H"# in thiophene. - Assume
#"100 g"# of thiophene to find the mol ratios. - Check with the molecular mass to see if the empirical formula is any different from the molecular formula.
In the first reaction:
#"7.96 g C"_x"H"_y"S"_z harr "16.65 g CO"_2#
In the second set of reactions:
#"4.31 g C"_x"H"_y"S"_z harr "11.96 g BaSO"_4#
where we assume thiophene was the limiting reagent...
This begins part
STEP 1: MASS PERCENT OF S
As the quantity of sulfur must obey conservation of mass, here is a nice trick we can use: we can convert from the mass of one substance to another using the ratio of their molar masses.
#"11.96 g " cancel("BaSO"_4) xx (32.065 cancel"g/mol" " S")/(233.38 cancel"g/mol" cancel("BaSO"_4))#
#=# #"1.643 g S"# that came from thiophene,assuming no other reactants contained sulfur.
That means we can use the thiophene mass from the second set of reactions to find the mass percent of
#"S"# in thiophene.
#(1.643 cancel"g" " S")/(4.31 cancel("g") " thiophene") xx 100% = color(green)ul(38.13%"w/w S")# in thiopheneSTEP 2: MASS PERCENT OF C & H
From the first reaction, notice that thiophene is the only source of carbon for
#"CO"_2# . Thus, we can similarly find the mass of#"C"# that is in#"CO"_2# , and by conservation of mass, that was the mass in thiophene.
#"16.65 g " cancel("CO"_2) xx (12.011 cancel"g/mol" " C")/(44.009 cancel"g/mol" cancel("CO"_2))#
#=# #"4.544 g C"# that came from thiophene.This means the mass percent of
#"C"# in thiophene is:
#(4.544 cancel"g" " C")/(7.96 cancel"g" " thiophene") xx 100% = color(green)ul(57.09% "w/w C")# in thiopheneThis leaves a hydrogen atom mass percent of:
#100.00% - 38.13% - 57.09%#
#= color(green)ul(4.78_7% "w/w H")# in thiophene(where the subscript indicates the digit past the last significant digit.)
STEP 3: MOL RATIOS & EMPIRICAL FORMULA
Currently our mass percents are only relative. If we choose a
#"100 g"# mass of thiophene, then in that mass, we have absolute masses:
#4.78_7# #"g H"# #"57.09 g C"# #"38.13 g S"# Now, if we have everything in mols, they can be compared in a ratio. So, divide by their molar masses to get the mol quantities of each:
#(4.787 cancel"g H")/(1.0079 cancel"g H""/""mol H") = "4.749 mols H"#
#(57.09 cancel"g C")/(12.011 cancel"g C""/mol C") = "4.753 mols C"#
#(38.13 cancel"g S")/(32.015 cancel"g S""/mol S") = "1.191 mols S"# We see that the mols of
#"H"# and#"C"# are about equal, and are also about four times the mols of#"S"# . To proceed, divide by the smallest mol quantity:
#(1.191 cancel"mols" "S")/(1.191 cancel"mols") = bb1# equiv. of#"S"#
#(4.753 cancel"mols" "C")/(1.191 cancel"mols") ~~ (4.749 cancel"mols" "H")/(1.191 cancel"mols") = 3.991#
#~~ bb4# equivs. of#"C"# and#"H"# eachSo, we construct an empirical formula of:
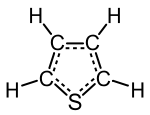
#color(blue)("C"_4"H"_4"S")# This is currently the most "reduced" form of thiophene's chemical formula, i.e. the mol ratios are as small as they can be with integer subscripts.
And now for part
STEP 4: MOLECULAR FORMULA
Knowing that its molecular mass is
#"84 amu"# , or#"84 g/mol"# , we compare with the molecular mass we would get using its empirical formula to see if we have the proper molecular formula yet.
#M_("C"_4"H"_4"S") = 4 xx "12.011 g/mol" + 4 xx "1.0079 g/mol" + "32.065 g/mol"#
#=# #"84.141 g/mol"# #~~# #"84 g/mol"# So, our empirical formula IS the molecular formula.