how are lithium atoms bonded together in the solid?
1 Answer
May 20, 2018
Through metallic bonding.
Explanation:
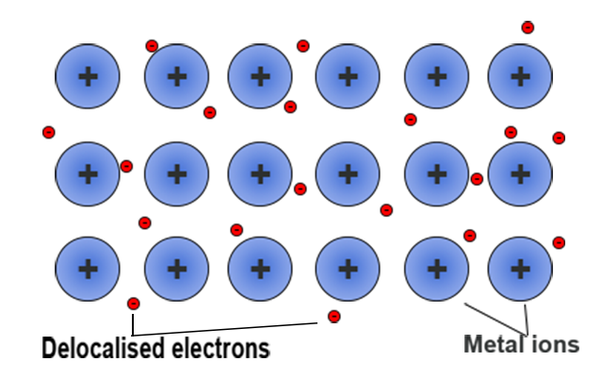
Lithium is a metal, so pure lithium metal would just have lithium atoms bonded together. The way they bond together is called metallic bonding, where lithium atoms release electrons into a "sea" and become cations, and bond with these spare electrons in the "sea", called delocalized electrons.
This forms a very strong lattice structure, due to the magnetic force from the opposite charges, and that is why metals have high boiling and melting points, as it is very hard to break those bonds.