How can I balance this equation? ____ KClO_3 -> ____ KCl + ____ O_2
2 Answers
You follow a systematic procedure to balance the equation.
Explanation:
Start with the unbalanced equation:
A method that often works is to balance everything other than
Another rule is to start with what looks like the most complicated formula.
The most complicated formula looks like
We put a
We can't change a coefficient unless we run into a roadblock (like having to use fractions).
My teacher never let me use fractions.
My solution when I hit a roadblock was to erase all the numbers and then start over again with a 2 as the starting coefficient.
We start with
Balance
We have 1
Balance
Balance
We have 3
We start over with a 2 as the coefficient.
Now we have 6
Every formula now has a fixed coefficient.
We should have a balanced equation.
Let’s check:
Left hand side: 2
Right hand side: 2
All atoms balance.
The balanced equation is
To balance a chemical equation is not different to solve a linear equations system. We assign a unknown variable to each of the coefficients of the reaction, and then solve the system.
Explanation:
Formally, the equation of a chemical reaction can be written as a mathematical equation that relates the number of atoms of each element involved in the reaction.
Because the atoms of the different elements are not transformed into each other (are talking about chemical reactions, not nuclear ones), the number of atoms of each element that is in the left side of the reaction (reactants) has to be equal to the number of atoms of the same element present on the right side of reaction (products).
Therefore, we can write a mathematical equation that equals both amounts. There will be as many equations as elements present in the reaction and have as many unknown variables as molecules of different substances involved.
Let's see how all this works with a real example. Consider the reaction that has been proposed in this question:
We calculate the coefficients of the reaction, ie numbers
Then we must write one equation for each element present in the reaction. There are three different elements in the reaction, so we have three equations:
Potassium :
Chlorine:
Oxygen:
In this example, we obtain a linear equations system with three unknown variables and only two equations, because two of them are the same one:
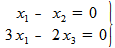
Obviously, it is a system of linear equations compatible but undetermined. We need to get only one solution (there are infinite ones).
Then, if we assign (arbitrarily)
Mathematically, the solution thus obtained and would be acceptable, but from a chemical point of view, it is inconvenient to use fractional coefficients. So what we do is multiply all the coefficients obtained by a number such that we obtain integer values. In this case, we multiply by 3, and obtain:


