How can I draw sigma bonds?
1 Answer
Dec 20, 2015
Sigma (
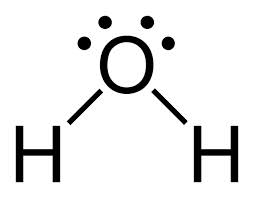
In a Lewis structure, a bond is often represented by a straight line:
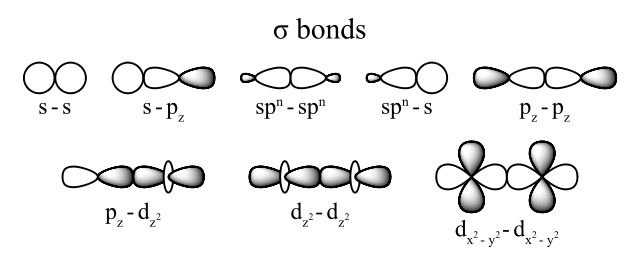
Sigma bonds when hybridized:
Sigma (
In a Lewis structure, a bond is often represented by a straight line:

Sigma bonds when hybridized:
