How can I draw the Lewis structure for #CO_2#?
1 Answer
You follow a sequence of steps.
Explanation:
Here are the steps that I follow when drawing a Lewis structure.
1. Decide which is the central atom in the structure. That will normally be the least electronegative atom (
2. Draw a skeleton structure in which the other atoms are single-bonded to the central atom:
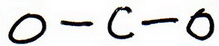
3. Draw a trial structure by putting electron pairs around every atom until each gets an octet.
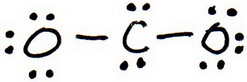
4. Count the valence electrons in your trial structure (20).
5. Now count the valence electrons you actually have available.
The trial structure has four electrons too many.
We need to insert either a triple bond or two double bonds.
6. Draw new trial structures, this time inserting the extra bonds.
There are three possibilities:
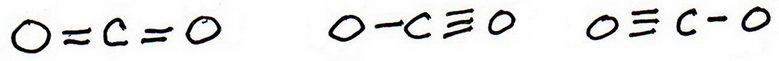
7. As before, add valence electrons to give each atom an octet:
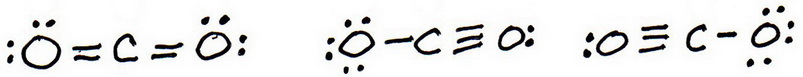
8. Calculate the formal charge on each atom.
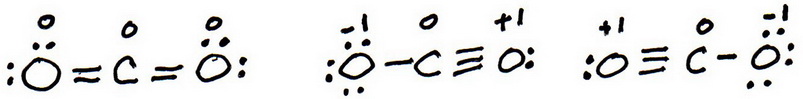
9. The “best” Lewis structure is one in which has the fewest formal charges.
The first structure has no formal charges, so the best Lewis structure for


