How can I draw the Lewis structure for NO?
1 Answer
You can find a procedure for drawing Lewis structures at
http://socratic.org/questions/what-is-the-lewis-structure-of-nh4br
For NO, the skeleton structure is N-O.
The trial structure is
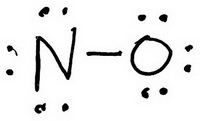
You have 14 valence electrons in your trial structure.
The valence electrons you have available are: 1 N + 1 O = 1×5 + 1×6 = 11. The trial structure has three extra electrons.
We have to insert one or more double bonds. With three electrons, we can make only one double bond with one electron left over: N=O.
With an odd number of electrons (11), we cannot give every atom an octet. We can write two possible structures.

The formal charge on each atom is:
Top structure: N = 5 - 3 - ½(4) = 0; O = 6 – 4 - ½(4) = 0
Bottom structure: N = 5 – 4 - ½(4) = -1; O = 6 - 3 + ½(4) = +1
The “best” Lewis structure is one that has the fewest formal charges — the top structure.
Thus, the Lewis structure of NO is


