How can the ionization energy of sodium be calculated knowing that the value of the wavelength at the Start of the continuum in the emittion spectrum is 242nm?
1 Answer
The ionization energy is
Explanation:
The value of the wavelength at the start of the continuum represents the ionization energy: the energy needed to remove a
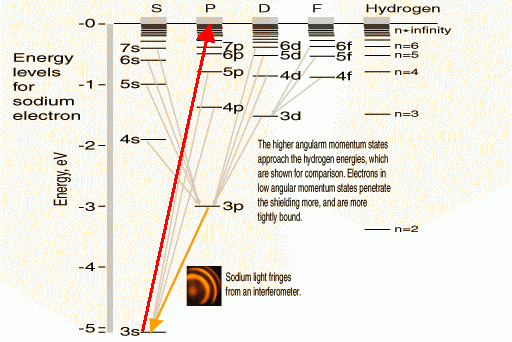 Na levels
Na levels
(Adapted from hyperphysics.phy-astr.gsu.edu)
The formula relating energy
color(blue)(bar(ul(|color(white)(a/a)E = (hc)/λcolor(white)(a/a)|)))" "
If
This is the ionization energy for one sodium atom.
For 1 mol of sodium atoms,

