How can you find heat of formation of water?
1 Answer
-280KJ
Explanation:
= -280KJ
The difference between
We have to calculate
For example
To calculate heat of formation we need a reaction that only forms water
[Bond energy of product that is
Bond energy of

Bond energy of O-H = 463KJ
No. of O-H bonds in water = 2
Bond energy of
There are 2 moles of water formed so bond energy will be twice
926KJ * 2 = 1852KJ
[Bond energy of products that is
Lewis dot structure of oxygen
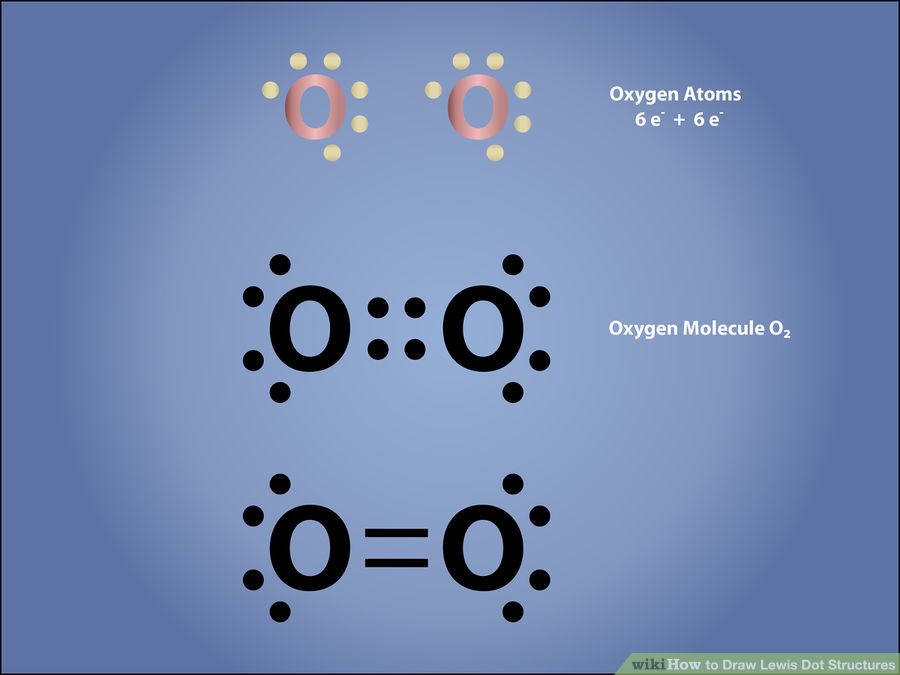
Bonds found in oxygen is 1 oxygen double bond
O = O = 499KJ
Lewis structure of Hydrogen
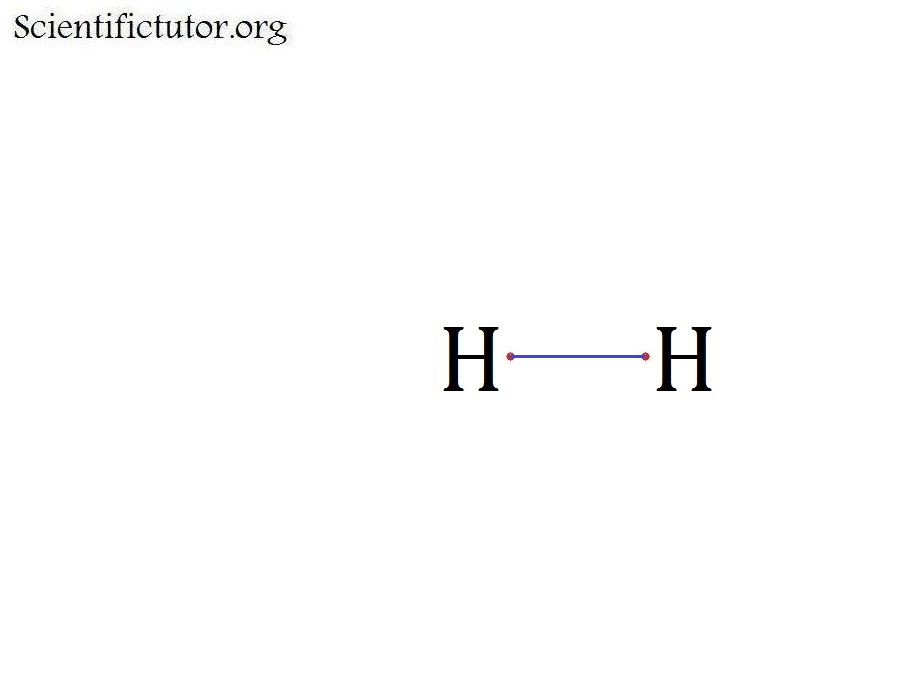
Bonds found in hydrogen is 1 H-H bond
H-H = 436KJ
There are 2 moles of hydrogen so the bond energy would be twice
436KJ * 2 = 872KJ
For the reaction
the
because all the products have become halve

