How can you tell the rate determining step?
1 Answer
Aug 19, 2016
When you first learn about it in high school, it is often just told to you which step is the slow (rate-limiting/rate-determining) step.
If you want to guess at which step it is, we assume you know the mechanism already. Often, the slow step is the step that is least favorable, so it takes a while for the fairly unstable intermediate to form before it gets propelled to form the product(s).
An example is the
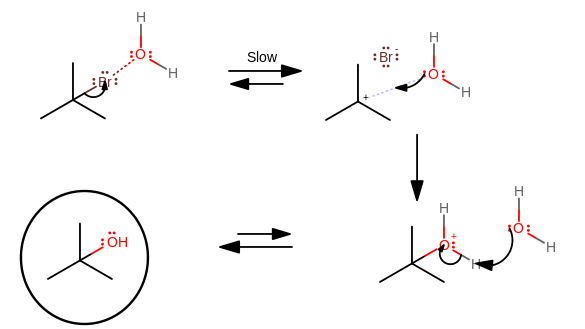
Attacking the carbocation is faster than forming it, because:
"Br"^(-) leaves on its own, when the nucleophile is slow. The slow nucleophile makes it so that"Br"^(-) has to participate by itself, i.e. in a first-order process, for this step, and thus"Br"^(-) has little to no assistance to leave faster.
Therefore, this is the slow step.
- The steric hindrance of tert-butyl bromide is lessened when the carbocation forms, since a planar molecule is inherently less sterically-hindered than its tetrahedral conformation.
Therefore, this is a faster step.

