How can you use electrical conductivity to decide if a compound is ionic or covalent?
1 Answer
Jan 29, 2017
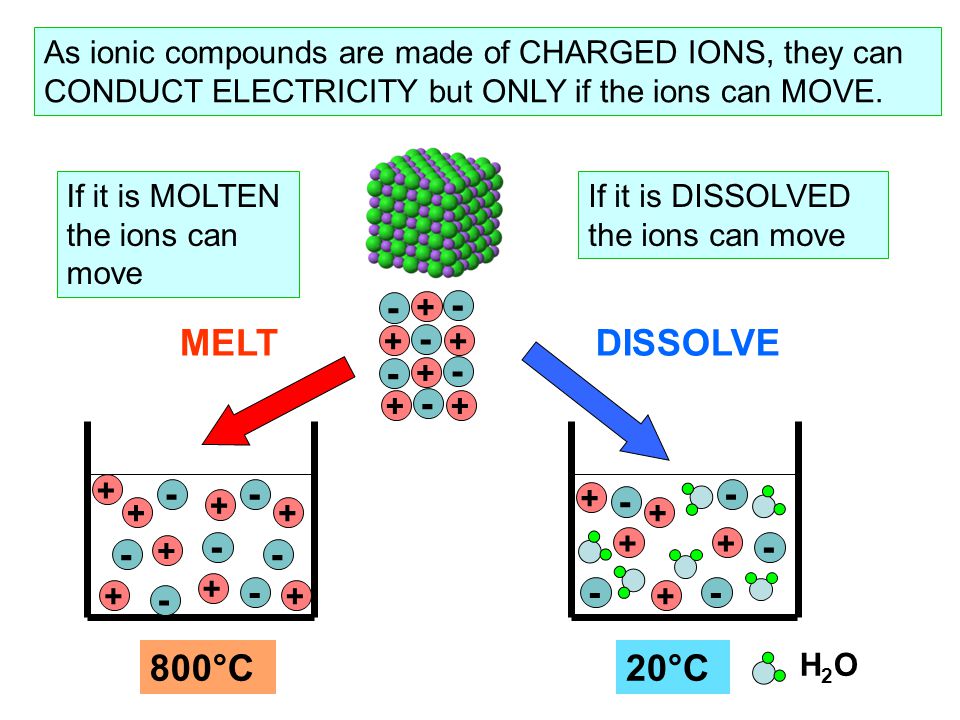
If a compound cannot conduct electricity in its solid or liquid state, it is covalent. If a compound does conduct electricity when dissolved in solution or molten, it is an ionic compound.
Explanation:
A covalent compound does not conduct electricity, either in its solid, liquid, or molten state.
Ionic compounds are able to conduct electricity only when their ions are free to move. This occurs when an ionic compound is dissolved in water, or when molten. When an ionic compound dissolves in water, the positive and negative ions dissociate and are then capable of conducting electricity. An ionic compound heated to its molten state will also conduct electricity because the ions dissociate and are free to move.