How did Bohr theory explain the emission spectrum of hydrogen?
1 Answer
By considering/imposing that energy is emitted when electrons "jump" from an allowed orbital to the next.
Explanation:
Bohr was faced by the big problem of a "planetary" like atom, where electrons orbit a central nucleus, where charged particles accelerated (centripetal acceleration) should emit continuously and fall into the nucleus; to solve this problem he postulated that electron stay on stable orbits where they do not emit energy.
The problem is now the opposite...what is with the energy emitted and visible in the emission spectrum?
He, again, postulated that electrons not only can 'jump" from a stable orbit to another but also that in doing so the exchange energy with the environment AND that the orbits they can use aren’t at random radial distances but at fixed (quantized) radial distances!!!
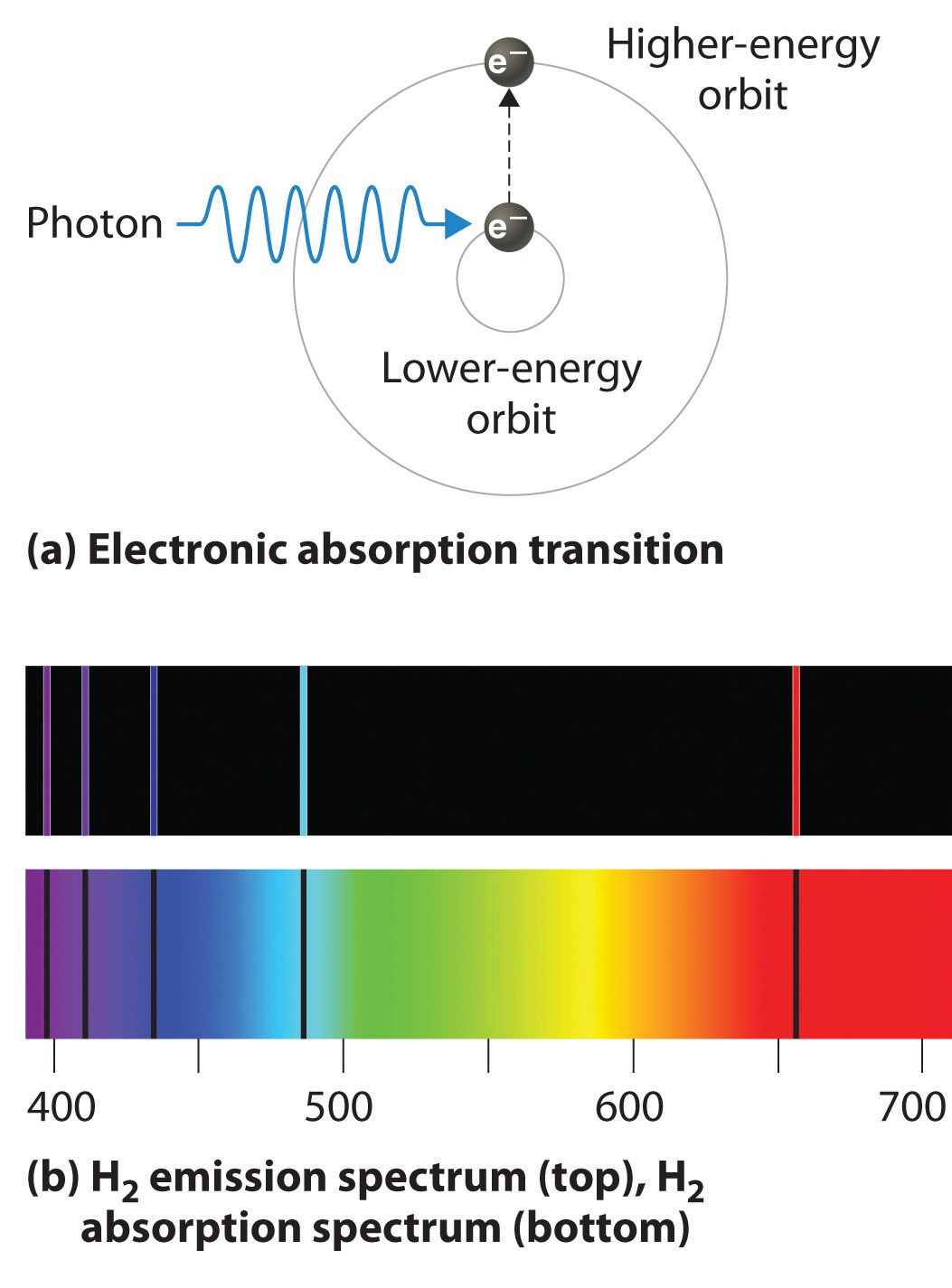
So, in essence, an electron stays in its lowest energy allowed orbit; the atom receives energy (the right amount) and so the electron can jump to the next allowed orbit that is at a fixed radial distance from the nucleus; the electron comes back to its lowest energy orbit releasing the energy it acquired previously.
The electron in hydrogen can only jump to fixed orbits and the energy that release going back forms the lines in our emission spectrum!!!
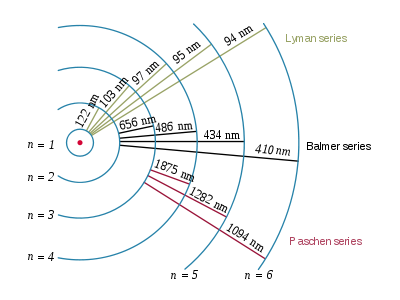
We can see this by considering that a photon emitted in these jumps will have a frequency
So, the lines in our emission spectrum are photons of a fixed amount of energy (dependent upon the orbit they correspond to) produced when an electron comes down from an allowed orbit!!!

