How do I balance long redox equations?
I can balance half and full redox equations but I struggle to balance an equation once other elements are thrown in between like I can balance the reduction and oxidation sides fine but what do I do with the original other elements which did not play a part in the two half equations but were there right at the start?
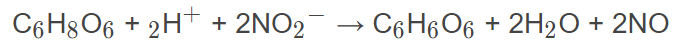
I can balance half and full redox equations but I struggle to balance an equation once other elements are thrown in between like I can balance the reduction and oxidation sides fine but what do I do with the original other elements which did not play a part in the two half equations but were there right at the start?
1 Answer
You assign oxidation states or average oxidation states to the original carbons...
Explanation:
You got
You finish with
Are you clear on how I assigned those numbers?
And so....
And meanwhile
And so we add
...and cancel away...
Does this address what you want? I am not entirely clear...

