How do isotopes differ from ions?
1 Answer
Jul 25, 2014
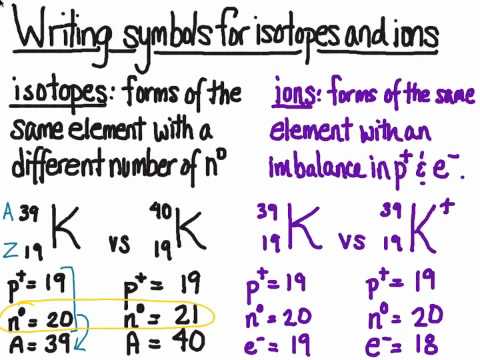
Isotopes differ in the number of neutrons; in ions the number of electrons is different from the number of protons.
Isotopes are atoms that have the same number of protons but different numbers of neutrons.
Thus, atoms of
Ions are atoms that have either gained or lost electrons.
Every atom has the same number of electrons and protons.
If an atom gains an electron, it gets a negative charge. If it loses an electron, it gets a positive charge. All ions have a charge.
If a carbon atom gains an electron, it becomes a C⁻ ion. If it loses an electron, it becomes a C⁺ ion.