How do molecules vibrate?
1 Answer
Dec 30, 2017
Naturally?
And they can do so by
- stretching (symmetrically/asymmetrically)
- bending (scissoring, wagging, twisting, rocking, or torsion motions)
and so on as follows:
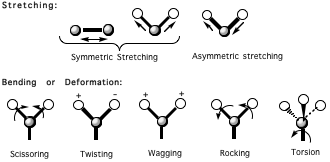
You can see GIFs of these motions here.
- The number of ways a linear polyatomic molecule can vibrate is
#3N - 5# . - The number of ways a nonlinear polyatomic molecule can vibrate is
#3N - 6# .
where
#N# is the number of atoms in the molecule.
Take formaldehyde as an example:
So we confirm that there should be
#3(4) - 6 = bb6# modes of vibration for formaldehyde,#"HCHO"# .
It isn't always the case that all of them are active in the infrared, but in this case they all are since formaldehyde is polar.
The arrows indicate which directions the atoms move for the particular mode of vibration. Note that the out-of-plane (OOP) bending has

