How do resonance structures affect molecular shape?
1 Answer
Sep 22, 2014
Resonance structures don't affect molecular shape, but they explain molecular shape.
For example, the Lewis structure of the carbonate ion CO₃²⁻ is

From this, we predict that it should have two C-O bonds of one length and a C=O bond of a shorter length.
Instead, we find experimentally that all the bonds are the same length.
We explain this by assuming that the CO₃²⁻ ion is a composite or resonance hybrid with three equivalent contributors.
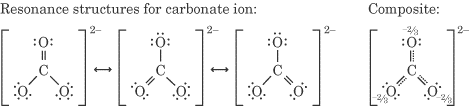
Each C-O bond in the resonance hybrid has exactly the same length.

