How do scientists use atomic spectra?
1 Answer
Scientists use atomic spectra to identify elements.
Explanation:
First of all, we need to know that electrons exist in energy levels that are quantised, or discrete:
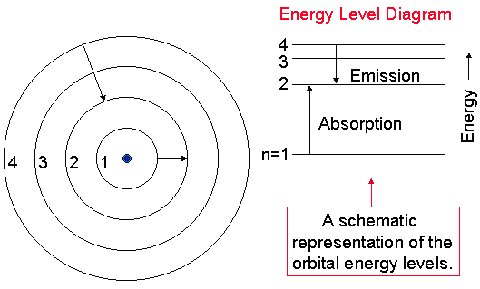
This means that, when electrons move from one energy level to another, only certain, specific amounts of energy is released.
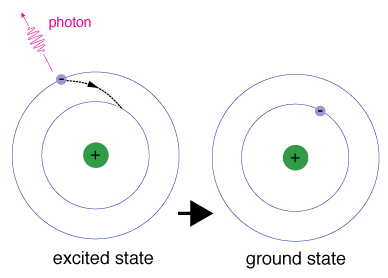
When we excite the electrons in an atom by giving energy, they will first move up from their initial, ground-state energy levels into higher energy levels.
Then, they will transition back down into their initial energy levels, with each electron releasing specific amounts of energy to do so.
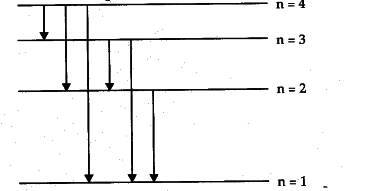
One way electrons release these specific amounts of energy is by emitting light, or photons.
As such, each energy level transition made by an electron will correspond to a photon with a specific amount of energy.
All of these photon energies are represented in atomic spectra.
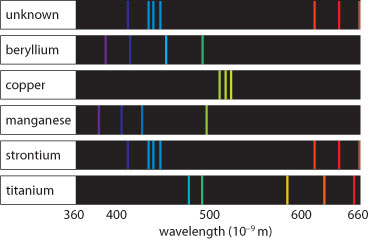
Atomic spectra are unique to each element—this is due to the energy level transitions being different for each element, basically because different elements have different numbers of electrons.
Therefore, atomic spectra can be used to identify elements.

