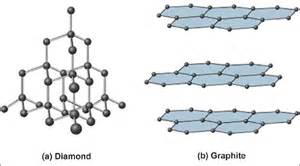
And #"graphite"# is non-molecular in two dimensions. The given figure illustrates this nicely.
Both solids are non-molecular with impossibly high melting and boiling points, but the difference in the degree of molecularity means that carbon graphite is relatively soft.
The #C-C# bond in diamond is approx. #1.54xx10^-10*m#, between individual carbon atoms. The corresponding distance in graphite is #1.42xx10^-10*m# between carbons in the same layer, and the shorter distance reflects some degree of electronic delocalization (as does its electrical conductivity). Between carbon layers the distance is approx. #3.35xx10^-10*m#. Diamond and graphite thus have different densities, #rho_"graphite"=2.27*g*cm^-3#, #rho_"diamond"=3.53*g*cm^-3#, and this difference reflects the distance between the layers in the graphite structure.