How do we use stable isotopes in science?
1 Answer
Biologists, archaeologists, geologists, and other scientists have many uses for the variations in the abundance ratios of stable isotopes.
Many elements have two stable isotopes. The lighter form is usually the more common one. It forms weaker bonds than the heavier one and reacts faster.
The slight differences in reactivity cause relative isotopic abundances to change over time. Different environments have predictable isotopic signatures. This change in isotopic abundance is called fractionation.
The isotopic ratios of stable isotopes are measured as deviations
where
Here are a few of the many uses of isotopic fractionation.
Carbon (
- Determining the routes of migratory plant-eating animals.
- Tracing the movements of migratory species between inshore and offshore.
- Determining shifts in diets.
- Reconstructing ancient diets.
- Locating the country of origin for a given explosive.
Nitrogen
- Determining dietary shifts of meat-eating animals.
- Distinguishing migratory routes in which animals feed from routes in which they do not feed.
- Positioning organisms in the food chain.

Hydrogen (
- Determining the breeding and wintering grounds of birds.
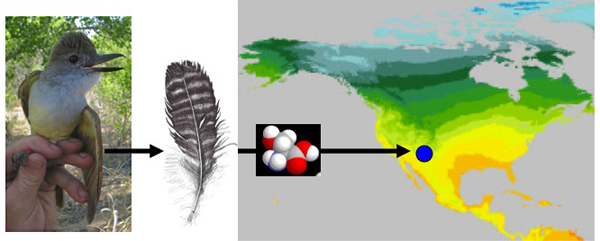
Oxygen (
- Tracking the movements of marine animals by the barnacle shells attached to them.
- Determining the geographic origin of paleodiets.
- Reconstructing climate change from ice and deep sea cores.

