How do you calculate the percent composition of water?
2 Answers
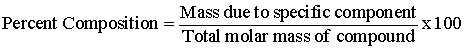
Explanation:
For water we have to calculate it % of Oxygen and % of Hydrogen
Its long calculation to do it here but this video will help
Since water is
Given that chemical formula, we have two equivalents of
The percent composition (by mass) of
#%"H" = (2xxM_"H")/(M_("H"_2"O"))xx100%#
#= (2xx"1.0079 g/mol")/("18.015 g/mol")xx100%#
#~~ color(blue)(11.19% "H")#
Similarly for
#%"O" = (1xxM_"O")/(M_("H"_2"O"))xx100%#
#= ("15.999 g/mol")/("18.015 g/mol")xx100%#
#~~ color(blue)(88.81% "O")#
Thus, the percent composition of water, by mass, is


