How do you convert #5.3 * 10^25# molecules of #CO_2# to moles?
1 Answer
Jun 23, 2016
There are 88 moles of
Explanation:
In order to go from molecules of 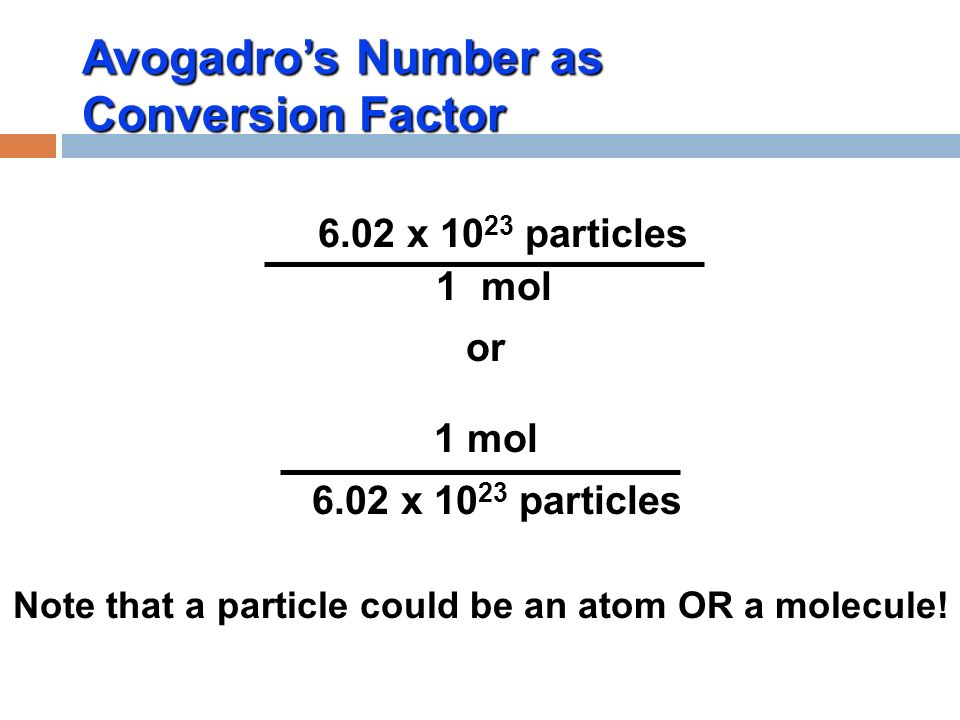
I usually set up dimensional analysis questions like this:
Quantity sought = Quantity given x conversion factor
- Quantity sought
#rarr# mol#CO_2# - Quantity given
#rarr# #5.3xx10^(25)# molecules - Conversion factor
#rarr# #(1mol)/(6.02xx10^(23))#
Now we just plug the values into the format I have above (make sure your units cancel out as you are going through this process, that's how you know if you are doing the calculations correctly or not):
moles
moles

