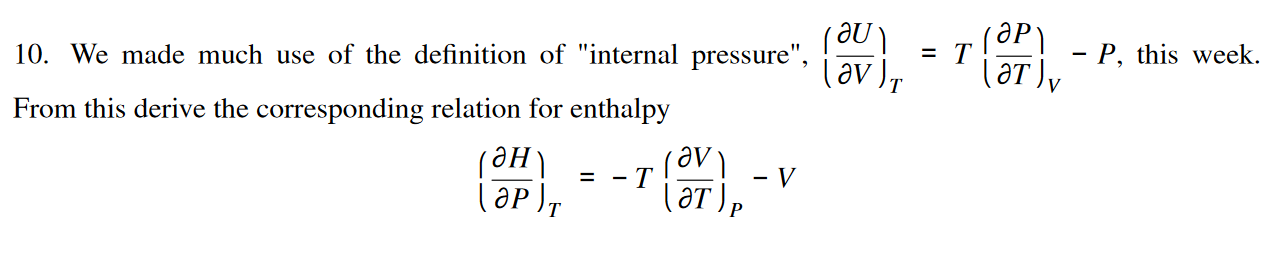
How do you derive this relation for enthalpy using this equation for internal pressure?
1 Answer
Well, we can derive it, but the final result we are "shooting for" is incorrect. It ought to give zero for ideal gases, but right now it won't.
We apparently have to begin with:
((delU)/(delV))_T = T((delP)/(delT))_V - P
Split the left-hand side:
((delU)/(delP))_T((delP)/(delV))_T = T((delP)/(delT))_V - P
Dividing the pressure derivative over, we obtain:
((delU)/(delP))_T = [T((delP)/(delT))_V - P] ((delV)/(delP))_T
Use the definition that
((delH)/(delP))_T - ((del(PV))/(delP))_T = [T((delP)/(delT))_V - P] ((delV)/(delP))_T
The left-hand side's second derivative uses the product rule to give:
((del(PV))/(delP))_T = P((delV)/(delP))_T + V((delP)/(delP))_T
= P((delV)/(delP))_T + V
Therefore, we get:
((delH)/(delP))_T - cancel(P((delV)/(delP))_T) - V = [T((delP)/(delT))_V - cancel(P)] ((delV)/(delP))_T
As a result, add the volume over to get:
((delH)/(delP))_T = V + T((delP)/(delT))_V ((delV)/(delP))_T
There is a cyclic relation that
-1 = ((delx)/(dely))_z((dely)/(delz))_x((delz)/(delx))_y
So if
-1 = ((delV)/(delT))_P ((delT)/(delP))_V ((delP)/(delV))_T
That means:
((delV)/(delT))_P = -1/[((delT)/(delP))_V ((delP)/(delV))_T]
= -((delP)/(delT))_V ((delV)/(delP))_T
Therefore:
color(blue)(((delH)/(delP))_T = - T((delV)/(delT))_P + V)
I've also derived this in another way, so we know this is correct.

