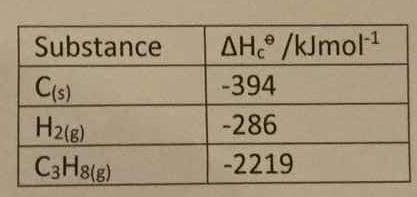
How do you determine the enthalpy change for the reaction below using the enthalpy of combustion data in the table: #3C_((s)) +4H_(2(s)) -> C_3H_(8(s))#?
1 Answer
You can do it like this:
Explanation:
The reaction in question refers to the enthalpy of formation of propane.
It is often very difficult to measure these experimentally since the reaction does not occur under standard conditions.
However, it is possible to burn both reactants and products and measure the standard enthalpy of combustion experimentally in a calorimeter.
The enthalpy of reaction can then be measured indirectly by constructing a Hess Cycle.
Hess' Law states that the overall enthalpy change of a reaction is independent of the route taken.
Write down the reaction you are interested in. Underneath write down the combustion products.
These will be the same since they are composed of the same elements.
Then construct the cycle using the data you have been given:
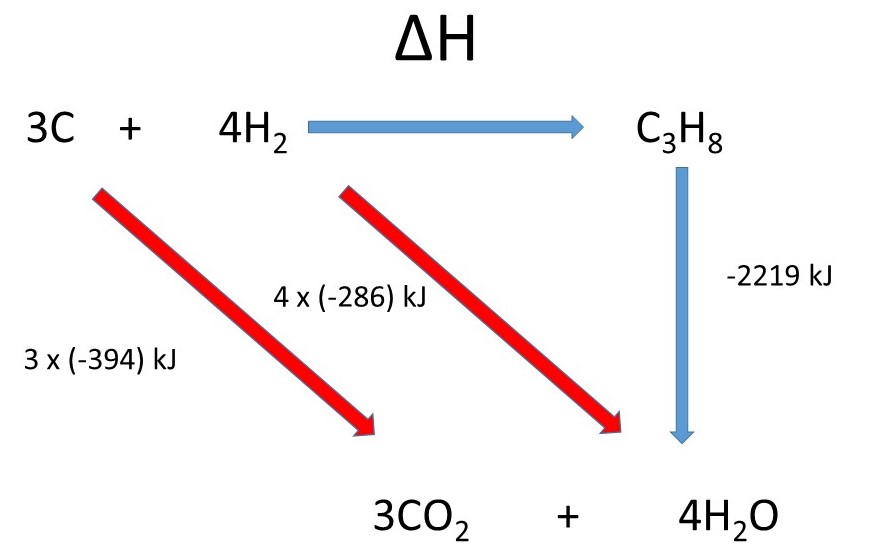
Applying Hess' Law we can say that, in terms of energy, the
This is because the arrows start and finish in the same place.
Note you must place the stoichiometric numbers in front of the values.
So we get:

