How do you draw VSEPR diagrams?
1 Answer
The Valence Shell Electron Pair Repulsion Theory (VSEPR) helps us to understand the 3D structure of molecules.
Explanation:
The general concept is that the pairs of electrons repel each other and try to locate themselves as far as possible from each other about a given nucleus.
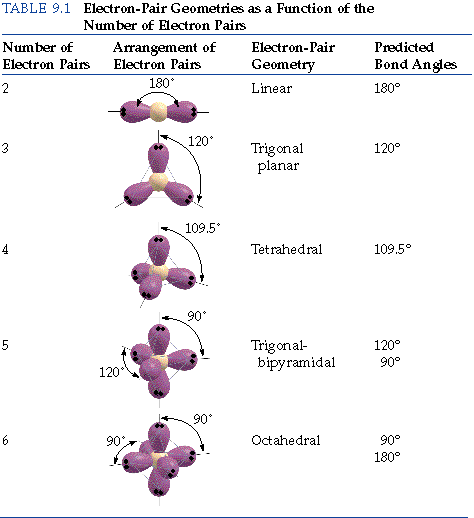
Hence, for two pairs of electrons on a nucleus, the two pairs would locate themselves exactly opposite each other, forming a bond angle of exactly 180°.
If three pairs exist, they will locate themselves in a plane about the nucleus at angles of 120° from each other.
Here is a table of the electron pair geometries as a function of the number of electron pairs.
To determine the shape of

The central atom,
The electron pair geometry of

The molecular geometry of
In determining the molecular shape, we consider only the positions of the atoms, not the lone pairs.
So, the molecular shape of

The lone pair of electrons occupies a relatively large volume, since they are held by only one atom.
They compress the bond angle between the oxygens and sulfur to less than 120°. The actual
Here is a table listing the molecular shapes that correspond to various combinations of bonding pairs and lone pairs.



