How do you find the atomic structure of an element?
1 Answer
See the explanation.
Explanation:
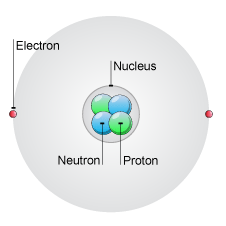
Atoms consist of a central nucleus containing positively charged protons,
An element's atomic number on the periodic table is the number of protons in the nuclei of its atoms. If the atom of an element is neutral, then the number of protons equal the number of electrons, which are located in the electron cloud surrounding the nucleus.
The number of neutrons in the nucleus in atoms of the same element can vary. Isotopes are atoms of the same element that differ in the number of neutrons.
So now you know that the nucleus of an atom contains protons and neutrons. The atomic number tells you the number of protons, and if the atom is neutral, the number of electrons, and that electrons are located in the electron cloud surrounding the nucleus.
If you know the atomic number of an element, you can determine the number of protons and electrons. If you know the isotope, you can also determine the number of neutrons.
The atom shown below is neutral. It has two positively charged protons, and two neutral neutrons inside the nucleus, and two negatively charged electrons surrounding the nucleus.