How do you find the equilibrium constant for the chemical equation?
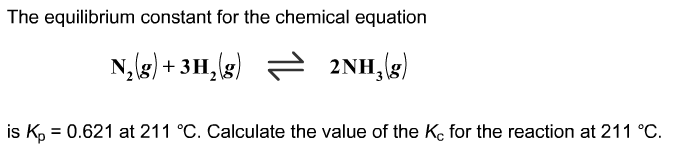
The formula is #K_p=K_c(RT)^(Δn)# but I'm not sure how to work this problem out
The formula is
1 Answer
Dec 4, 2017
A reaction at equilibrium,
has equal forward and reverse rates, and constant (but not equal) concentrations of reactants and products.
In this instance, we relate those variables with your equation to derive the equilibrium constant for concentrations from the equilibrium constant for pressures of that system. Hence,

