How do you identify aldehydes in skeletal structures?
Sometimes, when I try to name an aldehyde, I see it as a ketone and completely misinterpret the molecule. For example, in the molecule below, I see the double-bonded oxygen as a ketone, which would make sense normally. However, after I see that it is named as an aldehyde, I realize that the "methyl group" I thought was attached to the oxygen was actually CH, which would definitely be an aldehyde. THank
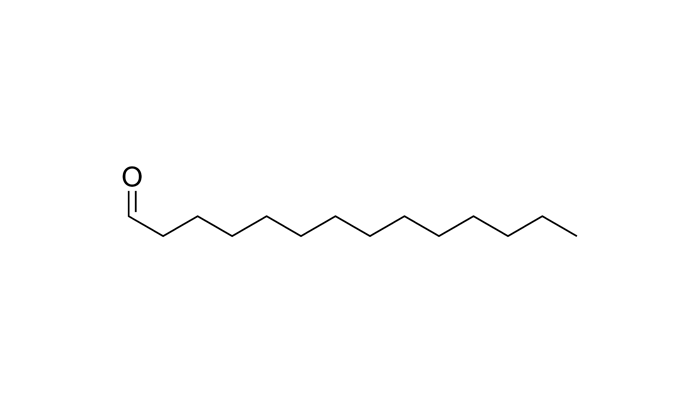
Thank you very much!
Sometimes, when I try to name an aldehyde, I see it as a ketone and completely misinterpret the molecule. For example, in the molecule below, I see the double-bonded oxygen as a ketone, which would make sense normally. However, after I see that it is named as an aldehyde, I realize that the "methyl group" I thought was attached to the oxygen was actually CH, which would definitely be an aldehyde. THank

Thank you very much!
1 Answer
Here's what I get.
Explanation:
A bond-line structure hides all
Each carbon atom must have four bonds, so any missing ones are
In your structure, the carbon on the far right has only one bond, so there must be three
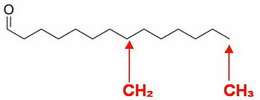
All the carbons at the angles of the zig-zag line show two bonds. They must also have two
Now, we examine the
The group is a
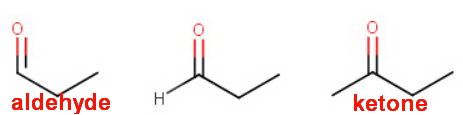
In the first structure, the carbonyl carbon has three bonds.
The fourth bond must be a
A ketone must have a

