How do you interpret an NMR spectrum?
What do the peaks represent? What different things affect the nature and positioning of the peaks (splitting, height, chemical shift, etc), and how do you interpret that into different elements/compounds etc?
Working in H1 spectra.
Thanks :)
What do the peaks represent? What different things affect the nature and positioning of the peaks (splitting, height, chemical shift, etc), and how do you interpret that into different elements/compounds etc?
Working in H1 spectra.
Thanks :)
1 Answer
Correctly....
Explanation:
See this old answer....
Any set of protons that can be interchanged by symmetry in a molecule are said to be to be chemically equivalent. And thus they give rise to ONE set of absorptions in the
So let us take ethane...
And now go to propane...
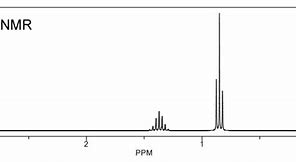
The integration has not been performed here, BUT INTEGRATION should be the first thing you should do to assign the spectrum.
What about
And so take the molecule you analyze. and predict the number of chemically equivalent protons....
So try this one....for

